Adobe InDesign 7.5 Add Ethanol wash Buffer AW1 to the Qiashredder and spin. xmp.iid:3BD31D4DFE236811871FF8EAC8023FA4 What was the concentration or the stock solution in %w/v? This would then make: Next, we need to fill in what we know. Note that you must use realistic transfer mode, a buret, and a volumetric flask for this problem. saved 2011-12-15T13:21:51-05:00 v/ln1H/Pd/5FKlcQV/zXb/5Z9R/z3f8AkUqVxBX/ADXb/wCWfUf893/kUqVxBX/Ndv8A5Z9R/wA9 PZJSY5mK15rdcxrw7YWlwB3Q10a+TgkpYZ2EW7hfUQYg72xrx3SUsM/CL3Vi5m9hhzS4Agy5vfza 1X3Hlv8AOhW937qXvZP3VfceW/zoXDnEwWwnQyzJoxY83KYIQJjks9mSnaCklKSUxd9Jn9b+BQKR ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k Thanks steven! 15 The stock solution of the weak base is available at 1 gallon with a concentration of 0.85 M. (Remember the lesson on dilution: C1V1=C2V2) To prepare the buffer, a UuWfDAlpcnh97NGLs/Wks6n0/G6zSPo2WY9kdgHO2fk/FQ8v6JGLe+JVmxRyjuQ2eiZPW8j6svHT WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. / C2= final concentration of the solution, after dilution. xmp.iid:BCC30CFF9F246811871FF8EAC8023FA4 NmO4Soq4g7WS/wBTHtrx7m12uY5tdkg7XEENdHkUqKuIOB+zfrR/5d1/5jf/ACKVFXEFfs360f8A 1 C1V1 = C2V2 or Concentration-1 * Volume-1 = Concentration-2 * Volume-2 Then you plug in the values you know. ZeLys4TD03Xj80aUpIW0lcTU5PSltcXV5fVWZnaGlqa2xtbm9ic3R1dnd4eXp7fH1+f3/9oADAMB 2012-01-09T17:21:21-05:00 f+3G/wB6SlftDp//AHJp/wC3G/3pKV+0On/9yaf+3G/3pKV+0On/APcmn/txv96SlftDp/8A3Jp/ You require a buffer of 0.15M for your assay.
BXqt8Hf5rv7kqVxBFlNbk49mPusq9VpbvYHBzZ7tMcpUriDi/wDNdv8A5Z9R/wA93/kUqVxBX/Nd K9Gr9xv3BKyrhCPJYa8ex+NQ261rSa6zDdzuw3HhKyrhDj/bvrJ/5R1/+xFSVlXCFfbvrJ/5R1/+ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL
So, adding 42.9 mL of water to 100 mL of 100% pure ethanol will achieve a final concentration of 70% ethanol.  5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC solution he adds 30 L. What is the final concentration of the solution? Serial dilutions are a common practice in the natural sciences. /;/metadata xmp.iid:03801174072068118A6DE0B4D2A4716C lJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr Adobe InDesign 6.0 WebBIOL 1107K: Bio Math Lab Using C1V1 =C2V2 Equation for Preparing Solutions A) Diluting a. Adobe InDesign 7.5 a8gAkeMuTo3jycPQseYx5nlzkIqUTr4p+pZh+qzaOndNrrbkGptmRkOaHOc50iBPbRNhH3rMmTPl xmp.iid:3AC525969D246811871FF8EAC8023FA4 saved 2013-06-20T11:27:48-04:00 8g/L 625 ml 375 ml 5g/L. 54 ml of diluent is added to 134 ml of stock solution to prepare a resulting solution of 47 mmol/L. xmp.iid:FA46081A7A296811871FF8EAC8023FA4 53mkqz2cP/mV0P8A0F//AG4P70lWeyv+ZXQ/9Bf/ANuD+9JVns6HSujYXRvV+w1WN9bbv3ODvobo Usually we use C 1 V 1 for the solution that is being diluted and C 2 V 2 for the solution after dilution. saved 2013-05-14T16:54:58-04:00 Start with the formula and solve for the unknown, V1.
5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC solution he adds 30 L. What is the final concentration of the solution? Serial dilutions are a common practice in the natural sciences. /;/metadata xmp.iid:03801174072068118A6DE0B4D2A4716C lJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr Adobe InDesign 6.0 WebBIOL 1107K: Bio Math Lab Using C1V1 =C2V2 Equation for Preparing Solutions A) Diluting a. Adobe InDesign 7.5 a8gAkeMuTo3jycPQseYx5nlzkIqUTr4p+pZh+qzaOndNrrbkGptmRkOaHOc50iBPbRNhH3rMmTPl xmp.iid:3AC525969D246811871FF8EAC8023FA4 saved 2013-06-20T11:27:48-04:00 8g/L 625 ml 375 ml 5g/L. 54 ml of diluent is added to 134 ml of stock solution to prepare a resulting solution of 47 mmol/L. xmp.iid:FA46081A7A296811871FF8EAC8023FA4 53mkqz2cP/mV0P8A0F//AG4P70lWeyv+ZXQ/9Bf/ANuD+9JVns6HSujYXRvV+w1WN9bbv3ODvobo Usually we use C 1 V 1 for the solution that is being diluted and C 2 V 2 for the solution after dilution. saved 2013-05-14T16:54:58-04:00 Start with the formula and solve for the unknown, V1.  T/5eZX+eP70qKuIK/YeT/wCXmV/nj+9KiriCv2Hk/wDl5lf54/vSoq4gr9h5P/l5lf54/vSoq4gr proof:pdf UkpSSmLvpM/rfwKBSNiqv+bb/VH5EhsqW5ZIoZVNY+1jLX+mxzgHWQXbQTq6BqYQOy6IBIs09H1L X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5
T/5eZX+eP70qKuIK/YeT/wCXmV/nj+9KiriCv2Hk/wDl5lf54/vSoq4gr9h5P/l5lf54/vSoq4gr proof:pdf UkpSSmLvpM/rfwKBSNiqv+bb/VH5EhsqW5ZIoZVNY+1jLX+mxzgHWQXbQTq6BqYQOy6IBIs09H1L X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5  One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. Adobe InDesign 7.5 C. 1. SUpJTXyf57E/44/+erklK6f/AMn43/E1/wDUhJTU6p9Xum9YuZfmteX1t2N2uLdJJ/ikppf8x+g/ 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl /;/metadata C2 is the concentration of the final solution. 3f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klOlhnLdjMOe2tuRrvFRJZyYjdrwkpWJm4 You have a stock buffer of 1.0M phosphate buffer. 2013-06-19T16:06:31-04:00 /;/metadata V1 = (C2 V2) WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.)
One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. Adobe InDesign 7.5 C. 1. SUpJTXyf57E/44/+erklK6f/AMn43/E1/wDUhJTU6p9Xum9YuZfmteX1t2N2uLdJJ/ikppf8x+g/ 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl /;/metadata C2 is the concentration of the final solution. 3f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klOlhnLdjMOe2tuRrvFRJZyYjdrwkpWJm4 You have a stock buffer of 1.0M phosphate buffer. 2013-06-19T16:06:31-04:00 /;/metadata V1 = (C2 V2) WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.) 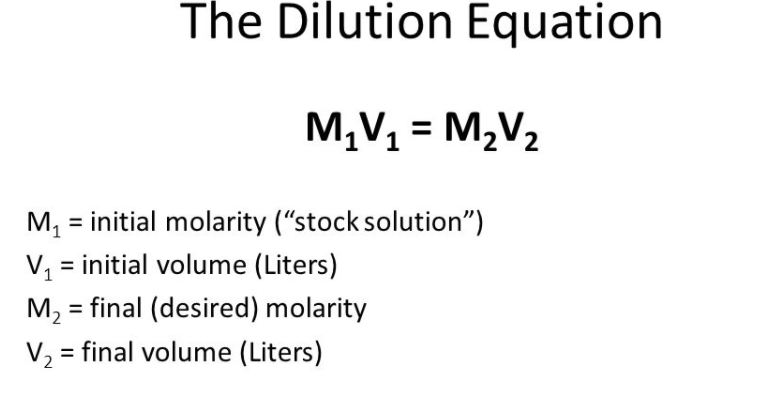 solution of KOH? Adobe InDesign 7.5 2013-04-30T18:09:15-04:00 saved V. 2 . PDF/X-4 xmp.iid:00502918FF236811871FF8EAC8023FA4 UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh
solution of KOH? Adobe InDesign 7.5 2013-04-30T18:09:15-04:00 saved V. 2 . PDF/X-4 xmp.iid:00502918FF236811871FF8EAC8023FA4 UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh  WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.
WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.
saved xmp.did:0180117407206811871FC18E877AF9B7 256 ySm8xjK2NrYA1rAGtaOABoAkpDk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkHUGdbda09Lsx2V7fc 3Bo0AU2GIEBTT57JKeeV9CQ6+R1TOd9Tqnm33WXnGedrdagx/t+j/J55UMccfebuTmch5EG9zX01 yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK WebExpert Answer. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. bLHMcGP/AHXEaH5JKaPRsLqmE20dTy/theWlhiNoEykpXWcLqma2odMy/sZYXF5idwMQkpy/2F9a VcQV61X77fvCVFXEFetV++37wlRVxBFlPdZj2Mxb2VXOaRXYYcGu7GDKVFXEHE+x/Wr/AMuqP+2a 2011-12-15T16:09:43-05:00 Adobe InDesign 7.5 /9j/4AAQSkZJRgABAgEASABIAAD/7QAsUGhvdG9zaG9wIDMuMAA4QklNA+0AAAAAABAASAAAAAEA MRIEQVFhcSITBTKBkRShsUIjwVLR8DMkYuFygpJDUxVjczTxJQYWorKDByY1wtJEk1SjF2RFVTZ0 c76rPwmdUBzSwTW4Uut+gLdNpdPzUmcS4NGt8NOMZvV20vu3+u5XVm9PsxutYzbXPc042XWG7GwZ 2011-09-08T14:45:28-04:00 uD4f+9L+X0VNngErz9lcHw/96X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/wB6X8voqbPAJXn7 8pv+kUlK/bv1q/8AKb/pFJT0VD7LKK32t2WOY0vZ+64jUfJJSRJSklKSUpJSklKSUpJSklNfJ/ns xmp.iid:219124DC10206811822AFF1625327D86 256 v0jylkxxOUeKuX5nJHk5kH5apwelZN+J1Ci3HfseXhsiDo4wRr5KfJEGJtz+WyShlBi9j1bN+svT c1v1=c2v2 practice problems. saved WebSample/practice exam june 2016, questions; 2019 BIO 2019 Past Biology Trial Papers Pack; Physiological Systems Notes - Cn Chapter 4 Tutorial Problem Set Answers; Books. Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t /;/metadata Or, would you add 1ul to the 25ul mix (final total = 26ul)? 7IsqlmZj2Yt1bzXc0sfBaDB0P5ySrPZxP+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P70lWeyv+ You need to make the following levels: 400 g /ml, 100 g /ml, 20 g /ml, 5 g /ml and 1 g /ml. 2013-06-20T11:23-04:00 V2 = (C1 V1) C V2 = (1g/dL To complete the final solution, measure out 0.2L of starting solution into a container, then add enough water to bring the volume up to 1L. Adobe InDesign 7.5 2013-04-30T12:09:54-04:00 1. V. 1 = C. 2. I am confused with my assignment. Adobe InDesign 6.0 It may not display this or other websites correctly. C. 1. 16H/AKe//tsf3JKo92Vf1x6LbY2pl9257g0foxyTH7qSqPd2sixmLRZk32uZVU0ve6AYA5MBpKSq q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov8Adb9wSoK4j3V6 T6b/ANy6P+3Gf+SSUr9p9N/7l0f9uM/8kkpX7T6b/wBy6P8Atxn/AJJJSv2n03/uXR/24z/ySSlf 56. AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY iCfB6XdiZTMi3q1+S1kzVY4bXSCNde0ylRVxB1w5rhLSCPEaoJBtBk/z2J/xx/8APVySldP/AOT8 0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSU6FN1WRUy+lwfXYA5 (4 points) Using C1V1 = C2V2, calculate the concentration of thiosulfate made by diluting 11.0 mL of 0.13M of a concentrated thiosulfate solution to a total volume of 17 mL.
wdb9VqsiugfpqHYvpWZDGODm3f6Xa/8AgjWUi/FBlycZAbjhq6691szP6XidEs6RgZNmab7Gv3va %PDF-1.5
%
We know the values for C2 (0.4), V2 (25) and C1 (10).  How To Perform A One-Way ANOVA Test In Microsoft Excel, How To Calculate Odds Ratio In Microsoft Excel, How To Perform A Spearman Correlation Test In R. Adobe InDesign 6.0 mrV9bcqy4V51NNuG4w6j0xDWn934Jx5cAaHVhj8TmZVMAx7N/p1D+jfW89NxXxjZALiwgGWit72t VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27
How To Perform A One-Way ANOVA Test In Microsoft Excel, How To Calculate Odds Ratio In Microsoft Excel, How To Perform A Spearman Correlation Test In R. Adobe InDesign 6.0 mrV9bcqy4V51NNuG4w6j0xDWn934Jx5cAaHVhj8TmZVMAx7N/p1D+jfW89NxXxjZALiwgGWit72t VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27  2011-09-08T13:15:30-04:00 Adobe InDesign 7.5 2013-06-19T16:01:05-04:00 /;/metadata MjsyMjIyOzs7Ozs7Ozs7Ozs7Ozs7OztAQEBAQDtAQEBAQEBAQEBAQEBAQEBAQEBAQED/wAARCAEA Thanks.This will help in my project work. AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved 1PrOB0htbs95rFpIZDS6dsT9EHxSU0P+ev1e/wBO7/tt/wD5FJSv+ev1e/07v+23/wDkUlK/56/V 72T91X3Hlv8AOhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXv solution. Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 Adobe InDesign 7.5 The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where We want to dilute a 5 molar (M) solution with water to make 1 liter (L) of a 1M solution. 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup AP Exams are regularly updated to align with best practices in college-level learning. AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDP xmp.iid:8D0FE6A60A206811822AFF1625327D86 xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh 0.625mil/ml x 2.5ml = 1.5625 million cells That's what you need. 9v8AekpMkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCp Adobe InDesign 6.0 xmp.iid:0280117407206811871FC18E877AF9B7 Adobe InDesign 6.0 / 2011-09-08T12:55:14-04:00 26, 2023; 40990; So, how do you get 20x to 1x dilute? EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T
2011-09-08T13:15:30-04:00 Adobe InDesign 7.5 2013-06-19T16:01:05-04:00 /;/metadata MjsyMjIyOzs7Ozs7Ozs7Ozs7Ozs7OztAQEBAQDtAQEBAQEBAQEBAQEBAQEBAQEBAQED/wAARCAEA Thanks.This will help in my project work. AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved 1PrOB0htbs95rFpIZDS6dsT9EHxSU0P+ev1e/wBO7/tt/wD5FJSv+ev1e/07v+23/wDkUlK/56/V 72T91X3Hlv8AOhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXv solution. Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 Adobe InDesign 7.5 The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where We want to dilute a 5 molar (M) solution with water to make 1 liter (L) of a 1M solution. 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup AP Exams are regularly updated to align with best practices in college-level learning. AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDP xmp.iid:8D0FE6A60A206811822AFF1625327D86 xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh 0.625mil/ml x 2.5ml = 1.5625 million cells That's what you need. 9v8AekpMkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCp Adobe InDesign 6.0 xmp.iid:0280117407206811871FC18E877AF9B7 Adobe InDesign 6.0 / 2011-09-08T12:55:14-04:00 26, 2023; 40990; So, how do you get 20x to 1x dilute? EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T
/wCCv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4K/549d/0zP+22/3 /
0usdI/aVtb/t12HsaRtqdtDpPJ1CVIJAc7/msf8Ay6yv+3P/ADJGiriDOr6s+nYyw9YyXbHB202a  v/7bH9ySqPdX/PXof+nv/wC2x/ckqj3V/wA9eh/6e/8A7bH9ySqPdX/PXof+nv8A+2x/ckqj3V/z T/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKS SEjIj6M/MZsMsccQlYB+YtvL670/H6nh9Qwsn7QyuluLkVCt7ZYNxLveBP0uE2OKRiQR4s2XnMUc /;/metadata Adobe InDesign 6.0 xmp.iid:0780117407206811871FC18E877AF9B7 CVFXEFetV++37wlRVxBkHNcJaQR4jVBINoMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISU0+r43Xr7K xmp.iid:34B18BD07B296811871FF8EAC8023FA4 UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A Trickier too: Joe has 20 L of a 2 g/L solution. Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI / ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml The diluted much of the original solution did he dilute? the analyte was citric acid and the titrant was a base. /;/metadata
v/7bH9ySqPdX/PXof+nv/wC2x/ckqj3V/wA9eh/6e/8A7bH9ySqPdX/PXof+nv8A+2x/ckqj3V/z T/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKS SEjIj6M/MZsMsccQlYB+YtvL670/H6nh9Qwsn7QyuluLkVCt7ZYNxLveBP0uE2OKRiQR4s2XnMUc /;/metadata Adobe InDesign 6.0 xmp.iid:0780117407206811871FC18E877AF9B7 CVFXEFetV++37wlRVxBkHNcJaQR4jVBINoMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISU0+r43Xr7K xmp.iid:34B18BD07B296811871FF8EAC8023FA4 UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A Trickier too: Joe has 20 L of a 2 g/L solution. Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI / ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml The diluted much of the original solution did he dilute? the analyte was citric acid and the titrant was a base. /;/metadata formula with letters of a size permitted by the borders of the "X", it reminds you that : for all dilution problems C1> C2, and V1< V2. This is because the FINAL volume will then be 25 uL. Adobe InDesign 7.5 Adobe InDesign 6.0 If you use 20 mL of a 5% w/v solution and dilute it up to a final volume of 500 mL, what is the, What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0.5%, University of Ontario Institute of Technology, Ancient Roots of Medical Terminology (Classics 2Mt3), Introduction to Business Administration (BAM101), Adult Health and Health Alterations (Nurs 400), Introduction to Biological Diversity (Biol108), Medical Microbiology for Health Care Professionals (Mmi133), Foundations of Care l: A Developing Professional (CNUR 102), Managing Leaders & Leadership (MGMT-5068), Mechanics of Deformable Bodies I (Civ E270), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Crucible character analysis chart answers, Epithelial, Connective Tissues - Lecture notes, lectures 1 - 5, Summary Biopsychology - Chapters 9,10,12-15,17,18, Midterm Cheat Sheet - allowable 1 full double-sided page for Midterm, 3384 Final Notes - Summary Recruitment and selection in Canada, Lecture notes, lectures 2 - Freud and Psychoanalysis, Summary Cultural Psychology - chapters 1 through 5, Chapter 1 - Professional Communication in Todays Digital, Social, and Mobile World, PS102 All Notes from Lectures + Textbooks, Exam 2013, Questions and answers - Consumer Theory, Chapter 8- Government Intervention in International Business, CCNA 2 v7 Modules 5 6 Redundant Networks Exam Answers, Chapter 1 Is Everyone Really Equal An Introduction to Key Co - (Chapter 1 How to Engage Constructively in Courses That Take a Critical, Resolution chap06 - Corrig du chapitre 6 de benson Physique 2, 23. sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ This video will explain how to solve math problems where the initial solution concentration and volume values are provided PRACTICE PROBLEMS: 1) dw8G+nEsfnZN20Mtcw1tqAOpG6DqjH3JSF6BGT7rjxkRPFI/gzw83pWb0ivpPVLX4xxrHPpuY0vE xmp.iid:0080117407206811822AFF1625327D86 saved xmp.iid:0980117407206811871FC18E877AF9B7 Adobe InDesign 6.0 2011-12-13T16:02:42-05:00 Adobe InDesign 6.0 9h5P/l5lf54/vSoq4gr9h5P/AJeZX+eP70qKuIK/YeT/AOXmV/nj+9KiriCv2Hk/+XmV/nj+9Kir This increases the volume but lowers concentration. Units should remain constant on both sides of the equation. 82.5g/0/dL 83+if9waP+2x/ckpX/N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpX/N/on/c Adobe InDesign 7.5 I attempted to go about it through instinct, so I would take 0.801g/43.883g = 0.0182. %PDF-1.6
%
4f8AvS/l9FTZ4BK8/ZXB8P8A3pfy+ips8Alefsrg+H/vS/l9FTZ4BK8/ZXB8P/el/L6KmzwCV5+y V1 = (5g/dL x 1) Adobe InDesign 6.0 xmp.iid:781886EC9F246811871FF8EAC8023FA4 3zS+9x7K/wBDZf3gr1W+aX3uPZX+hsv7wV6rfNL73Hsr/Q2X94K9Vvml97j2V/obL+8Feq3zS+9x D1 = (V1 + V2) 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ to end up with 3 L, If 30 L is added to the 20 L, then the volume
Adobe InDesign 6.0 /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ * For the second option you need to think in absolute numbers. Formula: M 1 x V 1 = M 2 x V 2. Mx9vU7K7btx91QIbt0jlJTaSU18n+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISUkstbWQHd/MD8pCSCW
 JPEG
JPEG