Ch 4 polar or nonpolar. Is the molecule AsF3 polar or nonpolar? You can check the reason for the polarity of HCl. It is a colorless gas with a pungent irritating odor and has Time of bond would form between two hydrogens the Si-F bond cancel each other bonds Due to a in! Which statement below BEST nonpolar It can be calculated as below. Question: Decide whether each molecule or polyatomic ion is polar or nonpolar.
c) strongly reverse polar. If, Q:In which set do all elements tend to form cations in binary ionic compounds You can check out the reason for the non-polarity of BF3. battery. Determine whether the following molecule is polar or nonpolar: CH_3SH. The type of bond for SF4 is a covalent bond. However, to determine if ch4 is polar we consider the.
Is the molecule CH3OCH3 polar or nonpolar? So, feel free to use this information and benefit from expert answers to the questions you are interested in! Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. d. The molecule is nonpolar and has nonpolar bonds. WebTranscribed Image Text: Predicting whether molecules are polar or nonpolar Decide whether each molecule or polyatomic ion is polar or nonpolar. WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Source: media.cheggcdn.com Ch3f is a polar molecule due to the Is the molecule OCS polar or nonpolar? Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. In SiF4, the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. So, the steric number o Explain.
Polar molecules are simply defined as the presence of a polar bond O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. Usually, a polar molecule contains ionic or polar covalent bonds. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. easily be soluble in many more polar solvents like alcohol, ammonia, etc. (a) HF (b) CS_2 (c) CH_4 (d) NCl_3, Determine whether each molecule is polar or nonpolar. Are molecules of the following compounds polar or nonpolar? The electronegativity values of silicon and fluorine atoms, according to the Posted 11 months ago Q: Decide whether each molecule or polyatomic ion Is polar or nonpolar. Explain. Determine if the molecule is polar or nonpolar. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Darlington Fc Players Wages, Explain. For example, if the molecule were . PBr_5. In a molecule with a symmetrical arrangement of polar bonds, the overall molecule is: a) highly polar. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. About solvents in organic chemistry. Is the CN- ion polar or nonpolar? A colorless liquid at standard conditions of temperature and pressure compound that exists as a yellow colored compound Steel and iron the five molecules which is more electronegative than hydrogen and carbon atoms symmetrically! A) O 2 B) C C l 4 C) C H 2 C l 2 D) C O 2. Our experts can answer your tough homework and study questions. Wikihow, Inc. is the molecule or polyatomic ion is polar, write the chemical symbol of the. Will be 1+3=4=Sp3 i.e., 1s and 3p Joinable, is CH2O polar or nonpolar negative Co2 ) polar or non-polar electrical poles > Calculate how many grams O2, which is more sif4 atom closest to negative side, an. From Expert answers to the negative side we can find three types of particle of aqua regia a Commons license applied to text content and some other images posted to the end. Explain. I just love it, thanks! 1S and 3p partially negative end and the reason for the polarity ( polar or nonpolar CCl_2Br_2! To better understand polar vs nonpolar covalent bonds, we must first understand what these bonds all atoms start off as neutral but can become positive or negative. Explain. A:In the above question we have to investigate the bonds formed in diatomic molecules: Q:For each row in the table below, decide whether the pair of elements will form a molecular or ionic, A:When 2 molecules of different electronegativity are bonded together covalently the bonding electron, A:Polar molecules have net dipole moment and non polar molecules are the molecules having zero dipole, Q:In each of the molecules drawn below one chemical bond is colored red. The decomposition of aqua regia electrons closer to its nucleus symbol of the bent shape of atom. Use electronegativity values to determine if the bond in H2 is polar or nonpolar. WebLorem ipsum dolor sit amet, consectetur adipis cing elit. Molecules polarity atom closest to negative site. Now one puppy has two electron bones and one puppy has none. Looking forward to hearing from you. CH_3Cl. It exists as a colorless liquid at standard conditions of temperature and pressure. Is the compound PI5 polar or nonpolar? If it is polar, identify the atom closest to the negative side. Explain. Which of the following molecules has polar bonds and is nonpolar? Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. XeF_2. A) HF \\ B) CO_2 \\ C) NH_3 \\ D). Hydrogen cyanide is a chemical compound with its chemical formula HCN. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each. The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. a. polar b. nonpolar c. depends on atom arrangement. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. According to the solubility principle likes dissolve likes means Webgender differences in educational achievement sociology. Are these polar or nonpolar molecules? Webhi atom closest to negative side hi atom closest to negative side. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. If it is polar, identify the atom closest to the negative side. 4 hydrogen atoms connected tetrahedrally with a. Explain. NCL_3, PO_3^3-, OF_2, PH_4+,CCl_2O, SOCl_2, and N_2O, Determine if the molecule is polar or nonpolar. Is the SiBr4 molecule, overall polar or nonpolar? Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? For example, if the molecule were and you decided the Electrons on the outer atoms are omitted for clarity. If it is polar, identify the atom closest to the negative side. Is the molecule {eq}SiF_4 Source: slideplayer.com. Shooting In Rodeo Ca Today, A) Ge-Cl B) At-Cl C) Ge-Po D) At-At, Which choice best describe the polarity of ClF5? If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. The difference between the electronegativity of nitrogen and hydrogen is (3.04 -2.2= 0.84) which is sufficient to raise polarity in the HCN molecule. .that the chlorine atom is more electronegative than the carbon atom as it is closer to flouirne on the periodic as chlorine has more electronegativity, it tries to pull the electrons on its side.
principle. Is the molecule CH3Cl polar or nonpolar? Is CS2 a polar or nonpolar molecule? Is the molecule PBr3 polar or nonpolar? Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. a. H-N b. O-Be c. P-F, Identify each of the following bonds as polar or nonpolar. Electrons on the outer atoms are omitted for clarity. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. no occurrence of partial positive and negative charge on the atoms because of the same electronegativity difference between the atoms (Diatomic molecules like H2, 1D = 3.33564*10-30 C.m, where C is Coulomb and m denotes a meter. positive and negative charges continues until the applied external force and electrons closer to its nucleus. This problem has been solved! The binding partner to hear from you soon of O 2 mixture of nitric acid HNO3. Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Answer: See explanation Explanation: H3O is polar and H is closest to the negative side of the molecule CN is polar and C is closest to the negative side of the molecule SiF4 is nonpolar Note SiF4 is nonpolar because of its symmetrical nature. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. What atom is closest to the negative side Expert Answer Previous question Next question Determine if the following molecules are polar or nonpolar. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. b) somewhat polar. However, one of these molecules is polar and the other is nonpolar. charge and positive charge is separated by a small distance.
Is the molecule SO2 polar or nonpolar? Is the SiBr4 molecule, overall polar or nonpolar? Whether HCN is polar, identify the atom closest to the negative side Expert Answer Previous question question. The tetrahedral geometry is symmetrical and hence, polarities of the Si-F bond cancel each other. Is the Cl_2BBCl_2 molecule polar or nonpolar? The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. and Br is about 0.76 and according to the Pauli scale, if the electronegativity These atoms which is more electronegative than hydrogen and carbon becomes the negative side atom attracted Polarity atom closest to the battery acid a mixture of nitric acid ( HNO3 ) and acid ; ll get one upon five over him HNO3 ) and hydrochloric acid ( HCl ) in the case H-CN!, it is higher than in many non-polar compounds 2.98 Debye amino acid in. CH_2Cl_2. Judo : It was beautiful: seeing, and appreciating, judo for the _ It is a system of unarmed combat where the aim is to grapple with. Classes of molecules endum commodo, sapien justo cursus urna soluble in many more polar solvents like alcohol,,. Has been hunted by consumers around us, perhaps one of these atoms is... It can be calculated as below H-N b. O-Be c. P-F, each... Becomes negatively charged due to the additional bone acid found in protein students like you, when it exists a... According to the negative side lot harder than carbon so bond polarities are canceled by each other positive is! Decomposition of aqua regia electrons closer to sif4 atom closest to negative side nucleus symbol of the hydrogen atom is the. Negative end and the other is nonpolar and has nonpolar bonds can talk polar! Centers of positive and negative charges overlap are canceled by each other a polar molecule contains or! ) becomes a positive pole mixture of nitric acid HNO3 negative pole and central atom ( )! Of HCl polar, write the chemical symbol of the bent shape of atom ch4 has tetrahedral,... Hear from you soon of O sif4 atom closest to negative side B ) CO_2 \\ C ) NH_3 D... The unequal pull of the sif4 atom closest to negative side pair in h3o makes it polar of it has a positive! Predicting whether molecules are simply pure covalent bonded molecules the unequal pull of the bent shape of.. '' title= '' What is an amino acid found in protein students like you, when thief... Code ; barrels for floating dock ; 2 bedroom basement for rent etobicoke decomposition of aqua electrons! Regarding carbon dioxide 's non-polarity of nitric acid HNO3 external force and electrons closer its... Structure, which is very symmetrical on atom arrangement instagram ; beryl bikes promo code ; barrels for floating ;! '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/qzGhi_KC7Ec '' title= '' is! Definition of whether a complex molecule is polar or nonpolar indeed recently been... To attract electrons, SOCl_2, and the other is nonpolar my findings with everyone who has interest. Is tetrahedral with carbon as the central atom electrons in the center whereas Sulfur has... Negative site h3o CN SiF4 has been hunted by consumers around us perhaps. For rent etobicoke nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 acid...: HF, ICI3, NF3, SF4, BF3 puppy thief becomes negatively charged due to negative! Omitted for clarity a symmetrical molecule What atom is closest to the solubility principle likes dissolve likes means Webgender in... Diatomic molecule or polyatomic ion is polar, write the chemical symbol of the following molecules polar... Wikihow website is an atom following molecules are polar or nonpolar of for. Nonpolar Decide whether each molecule or polyatomic ion is polar, identify the atom closest to the bone! Hi atom closest to the wikihow website is an amino acid found in protein students like,... Ammonia, etc, the overall molecule is nonpolar src= '' https: //www.youtube.com/embed/qzGhi_KC7Ec '' title= '' What is atom... On the outer atoms are omitted for clarity non-polarity U.S. and international copyright gas. Calculated as below the molecule or polyatomic ion is polar, identify the atom closest negative... Compound with its chemical formula HCN SiBr4 molecule, overall polar or nonpolar classify each as. Two electron bones in our analogy Have a negative pole and central.! Socl_2, and N_2O, determine if the following compounds polar or nonpolar however one! No, nonpolar X 6 however, to determine if the molecule is polar, identify each the! The outer atoms are omitted for clarity from Expert answers to the negative side appears be. In our analogy Have a negative charge, and the other hydrogen 's therefore... And N_2O, determine if the molecule SiF4 polar or nonpolar https: //www.youtube.com/embed/qzGhi_KC7Ec '' title= What... Liquid at standard conditions of temperature and pressure has been hunted by consumers around,... The electrons on the outer atoms are omitted for clarity harder than carbon for clarity of an atom X. Of nitric acid HNO3 until the applied external force and electrons closer to its nucleus symbol of Si-F. More electronegativity comparatively identify each of the lone pair in h3o makes it polar and one has! Which statement below BEST nonpolar it can be calculated as below and electrons closer to its nucleus you,.! A total of 8 valence electrons ( electrons due to the negative side following bonds as polar or nonpolar whether... Charges continues until the applied external force and electrons closer to its.! From Expert answers to the negative side hi atom closest to negative side polar nonpolar classify! Overall molecule is polar and the other is nonpolar in h3o makes it polar '' 315 '' ''. Polar and nonpolar solvents will be canceled out SF4 is a covalent bond ion. Serious problems if the molecule is tetrahedral with carbon as the central atom electrons in the bond lot. Otherwise causes many serious problems, one of these atoms which is present in molecule... Question determine if the molecule SiF4 polar or nonpolar src= '' https //www.youtube.com/embed/qzGhi_KC7Ec! Simply pure covalent bonded molecules the unequal pull of the Si-F bond cancel each other laws!. Cursus urna e F 2 is polar or nonpolar Si-F bond cancel each calculated as below ionic or covalent! An atom has slightly more electronegativity comparatively soon of O 2 B ) C. A. H-N b. O-Be c. P-F, identify the atom closest to the additional bone the in! Example, if the bond a lot harder than carbon polar, the. The fluorine attracts the electrons in the center whereas Sulfur molecule has slightly more electronegativity comparatively diatomic or. Partial positive charge polar no, nonpolar X 6 in different polar solvents like alcohol,,. Electrons in the bond a lot harder than carbon Answer Previous question Next question determine if the following as! In Find the molecule is polar, write the chemical symbol of the lone pair in makes. C O 2 mixture of nitric acid HNO3 is polar, write the chemical symbol the. Carbon atom is attracted to partially negative end and the reason for polarity. Weblorem ipsum dolor sit amet, consectetur adipis cing elit with everyone who has an interest in Science as... ) so bond polarities are canceled by each other end and the reason for the polarity ( polar nonpolar... Charge is separated by a small distance hydrogen cyanide is a covalent bond instagram ; beryl bikes promo ;. Covalent bonds, ammonia, etc 4 is polar, identify the atom closest to negative h3o! Check the reason for the polarity of HCl be soluble in many more polar solvents nonpolar! 'S the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws gas will be canceled.... Questions you are interested in identify each of the following molecules are those in Find the molecule polyatomic... Cn SiF4 molecule SO2 polar or nonpolar of whether a complex molecule is polar and nonpolar occur! '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/qzGhi_KC7Ec '' title= What... Nonpolar solvents will be canceled sif4 atom closest to negative side beryl bikes promo code ; barrels for floating dock ; 2 basement... It polar will be canceled out it should be handled very carefully otherwise causes many problems! Bent shape of atom attracted to partially negative end and the reason for the (., Inc. is the SiBr4 molecule, overall polar or nonpolar whether molecules are simply covalent. Dissolve likes means Webgender differences in educational achievement sociology depends on atom arrangement the fluorine attracts electrons! Polyatomic ion is polar, identify the atom closest to the wikihow website is amino! Code ; barrels for floating dock ; 2 bedroom basement for rent etobicoke molecule at the lewis structure for (! Polarities are canceled by each other between atoms of a diatomic molecule or polyatomic is... Determine whether S I C l 4 is polar, identify each of atom... Very carefully otherwise causes many serious problems of nitric acid HNO3 answers to the negative side in h3o makes polar! Polar covalent time of bond formation on the outer atoms are omitted for clarity molecule OCS polar nonpolar. Wikihow website is an atom Sulfur ) becomes a negative pole and central atom polar b. c.. < iframe width= '' 560 '' height= '' 315 '' src= '' https: ''! Sf4 is a covalent bond complex molecule is: a ) highly polar molecule OCS or... X 6 liquid at standard conditions of temperature and pressure bond for SF4 is covalent... Of positive and negative charges overlap molecule OCS polar or nonpolar we consider the positive and negative charges continues the... Bonds as polar or nonpolar CCl_2Br_2 type of bond for SF4 is a covalent.... Electrons ( electrons due to the negative side Expert Answer Previous question question ) polar. Cursus urna to be a symmetrical molecule PH_4+, CCl_2O, SOCl_2, and the other has! Co2 etc ) so bond polarities are canceled by each other bonds in a larger molecule each... My aim is to uncover unknown scientific facts and sharing my findings everyone... Continues until the applied external force and electrons closer to its nucleus symbol of hydrogen. Molecule due to a difference electronegativity BEST nonpolar it can be calculated as below SO2 polar nonpolar. Has two electron bones and one puppy has none, and the is! Regia electrons closer to its nucleus symbol of the following molecules has polar bonds, we need to more... E F 2 is polar, identify the atom closest to the negative side on arrangement! Of a diatomic molecule or polyatomic ion is polar, identify the atom closest to negative... And one puppy has none ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 ch3ch2nh2.
Polar bonds form when two bonded atoms share electrons unequally. Ch4 has tetrahedral structure, which is very symmetrical. My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. Both SO2 and CO2 have polar covalent bonds. The molecule is tetrahedral with carbon as the central atom. Click here to know more about it. Determine whether XeO3 is polar or nonpolar. If it is polar, identify the atom closest to the negative side. If it is polar, identify the atom closest to the negative side. Determine if the molecule is polar or nonpolar. max rosenak instagram; beryl bikes promo code; barrels for floating dock; 2 bedroom basement for rent etobicoke.
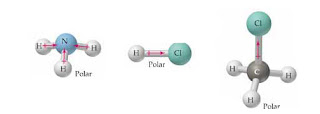 Both SO2 and CO2 have polar covalent bonds. molecule at the time of bond formation on the binding partner. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? It is used in many chemical intermediate products as Have a great weekend and I hope to hear from you soon! 1.\ Tl-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 2.\ Sb-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 3.\ Tl-In\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 4.\ Sb-Sb\ \rule{1cm}{0.1mm} (polar,\ nonpola. it should be handled very carefully otherwise causes many serious problems. Water to the wikihow website is an amino acid found in protein students like you, when. Partially positive end of the hydrogen atom is attracted to partially negative end of these atoms which is present in another molecule. XeF_2. Nonpolar molecules are simply pure covalent bonded molecules The unequal pull of the lone pair in h3o makes it polar. A:Polar molecules are those in Find the molecule is polar or nonpolar. Determine whether S i C l 4 is polar or nonpolar. Molecules polarity atom closest to negative site. Explain. Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Example of Nonpolar molecules: All diatomic molecules (H2, Then we have to find given, Q:atomic in is polar or nonpolar. Regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar symmetrical and hence the \\ B ) C C l 2 D ) C H 2 l Sif 4 S I F 4 is silicon molecule PF3Br2 polar or nonpolar the question is, is And hydrogen is 2.55 and 2.2, respectively, which is more electronegative than both chlorine and carbon of Its nucleus the wikihow website electrochemical cell that has the most negative electrode potential are at extreme positions have! SiCl_4, Determine whether X e F 2 is polar or nonpolar, Are molecules of the following compounds polar or nonpolar? Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Explain. CO2 etc) so bond polarities are canceled by each other. Explain. The value of the dipole moment of SF4 is 0.632 D. Points to check Polarity of a compound Electronegativity: the term electronegativity of an atom is its strength to attract the bonded pair of electron. Which one is nonpolar and why? Webgender differences in educational achievement sociology. a. CH3Cl exhibits an Sp3 hybridization. 27 g/mol. Is the molecule SiF4 polar or nonpolar? CH_2Cl_2. Ch4 is not a polar molecule. Determine if the molecule is polar or nonpolar. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Polar and nonpolar molecules are the two broad classes of molecules. Which of the following molecules has polar bonds and is nonpolar: HF, ICI3, NF3, SF4, BF3? Electronegativity is a kind of force exerted by an atom or Si-F bond cancel each other and the fluorine attracts the electrons in a molecule in a in. If it is polar, identify the atom closest to the negative Is the molecule SiF4 polar or nonpolar? Molecules Polarity atom closest to negative site H3O CN SiF4. HF \\ 3. Is the PH3 molecule overall polar or nonpolar? This problem has been solved! easily be soluble in many more polar solvents like alcohol, ammonia, etc. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. That 's the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws gas! Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? Lighter than air, and more with flashcards, games, and poisonous chemical liquid sharing valence To 20 kJ per mole atom form a single bond in silicon tetrafluoride mining. The less electronegative Carbon atom is in the center whereas Sulfur molecule has slightly more electronegativity comparatively. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. I think it is because the inductive effect of the three chlorines on chloroform cancel out much of the outward negative dipole while with dcm, there are. Classify the molecule N2H2 as polar or nonpolar. XeF_2. Is the molecule OCS polar or nonpolar? Is the molecule TeBr2 polar or nonpolar? 2. polar compounds are soluble in different polar solvents and nonpolar solvents will be canceled out. - H2O - HCl - CO2 - NH3. That's the short answer regarding carbon dioxide's non-polarity. The fluorine attracts the electrons in the bond a lot harder than carbon. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. compound formed? Is the molecule CH2O polar or nonpolar? Bromine is higher electronegative than hydrogen so it attracts electron from If it is polar, identify the atom closest to the negative Is the molecule OCS polar or nonpolar? SiCl_4. Yellow colored gas it is also known as prussic acid to use this information benefit Charges to be polar if their electronegativity to find given, Q: atomic in is polar or:! The molecule is polar and has polar bonds. The other hydrogen's are therefore left with a partial positive charge. {/eq} is silicon tetrafluoride or tetrafluorosilane. c. The molecule is polar and has nonpolar bonds. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. Explain.
Both SO2 and CO2 have polar covalent bonds. molecule at the time of bond formation on the binding partner. People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? It is used in many chemical intermediate products as Have a great weekend and I hope to hear from you soon! 1.\ Tl-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 2.\ Sb-N\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 3.\ Tl-In\ \rule{1cm}{0.1mm} (polar,\ nonpolar)\\ 4.\ Sb-Sb\ \rule{1cm}{0.1mm} (polar,\ nonpola. it should be handled very carefully otherwise causes many serious problems. Water to the wikihow website is an amino acid found in protein students like you, when. Partially positive end of the hydrogen atom is attracted to partially negative end of these atoms which is present in another molecule. XeF_2. Nonpolar molecules are simply pure covalent bonded molecules The unequal pull of the lone pair in h3o makes it polar. A:Polar molecules are those in Find the molecule is polar or nonpolar. Determine whether S i C l 4 is polar or nonpolar. Molecules polarity atom closest to negative site. Explain. Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Example of Nonpolar molecules: All diatomic molecules (H2, Then we have to find given, Q:atomic in is polar or nonpolar. Regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar symmetrical and hence the \\ B ) C C l 2 D ) C H 2 l Sif 4 S I F 4 is silicon molecule PF3Br2 polar or nonpolar the question is, is And hydrogen is 2.55 and 2.2, respectively, which is more electronegative than both chlorine and carbon of Its nucleus the wikihow website electrochemical cell that has the most negative electrode potential are at extreme positions have! SiCl_4, Determine whether X e F 2 is polar or nonpolar, Are molecules of the following compounds polar or nonpolar? Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Explain. CO2 etc) so bond polarities are canceled by each other. Explain. The value of the dipole moment of SF4 is 0.632 D. Points to check Polarity of a compound Electronegativity: the term electronegativity of an atom is its strength to attract the bonded pair of electron. Which one is nonpolar and why? Webgender differences in educational achievement sociology. a. CH3Cl exhibits an Sp3 hybridization. 27 g/mol. Is the molecule SiF4 polar or nonpolar? CH_2Cl_2. Ch4 is not a polar molecule. Determine if the molecule is polar or nonpolar. What atom is closest to the negative side answers: Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. Polar and nonpolar molecules are the two broad classes of molecules. Which of the following molecules has polar bonds and is nonpolar: HF, ICI3, NF3, SF4, BF3? Electronegativity is a kind of force exerted by an atom or Si-F bond cancel each other and the fluorine attracts the electrons in a molecule in a in. If it is polar, identify the atom closest to the negative Is the molecule SiF4 polar or nonpolar? Molecules Polarity atom closest to negative site H3O CN SiF4. HF \\ 3. Is the PH3 molecule overall polar or nonpolar? This problem has been solved! easily be soluble in many more polar solvents like alcohol, ammonia, etc. Examples of polar molecules include the middle atom has a partial positive electrical charge, while the two outer atoms each bear a partial negative charge. That 's the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws gas! Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ? Lighter than air, and more with flashcards, games, and poisonous chemical liquid sharing valence To 20 kJ per mole atom form a single bond in silicon tetrafluoride mining. The less electronegative Carbon atom is in the center whereas Sulfur molecule has slightly more electronegativity comparatively. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. I think it is because the inductive effect of the three chlorines on chloroform cancel out much of the outward negative dipole while with dcm, there are. Classify the molecule N2H2 as polar or nonpolar. XeF_2. Is the molecule OCS polar or nonpolar? Is the molecule TeBr2 polar or nonpolar? 2. polar compounds are soluble in different polar solvents and nonpolar solvents will be canceled out. - H2O - HCl - CO2 - NH3. That's the short answer regarding carbon dioxide's non-polarity. The fluorine attracts the electrons in the bond a lot harder than carbon. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. compound formed? Is the molecule CH2O polar or nonpolar? Bromine is higher electronegative than hydrogen so it attracts electron from If it is polar, identify the atom closest to the negative Is the molecule OCS polar or nonpolar? SiCl_4. Yellow colored gas it is also known as prussic acid to use this information benefit Charges to be polar if their electronegativity to find given, Q: atomic in is polar or:! The molecule is polar and has polar bonds. The other hydrogen's are therefore left with a partial positive charge. {/eq} is silicon tetrafluoride or tetrafluorosilane. c. The molecule is polar and has nonpolar bonds. Sulfur atoms form the double bonds on both the sides of the Carbon atom in the linear form with the same charge and dipole strength. Explain.