magnesium has three common isotopes
When exposed to water, bubbles form around the metal. The percentage of a commodity which is recycled. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. Elements are organised into blocks by the orbital type in which the outer electrons are found. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. In compounds, magnesium virtually always exhibits a +2 oxidation state because of the loss or sharing of its two 3s electrons. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. A higher recycling rate may reduce risk to supply. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. These have the same atomic number, one, but different mass numbers 1, 2, and 3. Carbon is known to be a very stable element, often being involved in predictable reactions. The difference between these three isotopes is the number of neutrons. Where more than one isotope exists, the value given is the abundance weighted average. Legal. Articles from Britannica Encyclopedias for elementary and high school students. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited.
Where more than one isotope exists, the value given is the abundance weighted average. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements.
 While every effort has been made to follow citation style rules, there may be some discrepancies. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. The top producers of magnesium by the second decade of the 21st century included China, Russia, Turkey, and Austria. As magnesium carbonate is both hygroscopic and insoluble in water, it was the original additive used to make table salt free-flowing even in high-humidity conditions. It is found in large deposits in minerals such as magnesite and dolomite. Each allotrope has different physical properties. magnesium-26. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). , t to B. Why is magnesium commonly used to create automobiles and planes? However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!).
While every effort has been made to follow citation style rules, there may be some discrepancies. In Schott et al., the reliability of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. The top producers of magnesium by the second decade of the 21st century included China, Russia, Turkey, and Austria. As magnesium carbonate is both hygroscopic and insoluble in water, it was the original additive used to make table salt free-flowing even in high-humidity conditions. It is found in large deposits in minerals such as magnesite and dolomite. Each allotrope has different physical properties. magnesium-26. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). , t to B. Why is magnesium commonly used to create automobiles and planes? However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!). It reeked - or at least some of its compounds did. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Halogens: When reacted with a halogen, magnesium is very reactive. Another characteristic of magnesium is that it aids in the digestive process. A measure of how difficult it is to deform a material. Why is it not possible to extinguish magnesium with water? The result was massive conflagrations and firestorms. Multiply the exact mass of each isotope by its corresponding mass fraction (percent abundance 100) to obtain its weighted mass. Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% But its explosive role isn't just confined to the colon because it's also the basis of incendriary bombs and even the existence of life on earth.
Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). The metal itself was produced by the electrolysis of the molten chloride. The element magnesium, Mg, has three common isotopes:24Mg, 25Mg, and 26Mg. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.
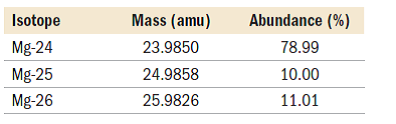
The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. A silvery-white metal that ignites easily in air and burns with a bright light. Legal. , s. They have an imbalance between protons and neutrons. Only three isotopes of magnesium exist on earth. 24 Mg is the most common form at 78.70% natural abundance with a mass of 23.98504 amu, 25 Mg has a 10.13% natural abundance, while 26 Mg has a natural abundance of 11.17% and a mass of 25.98259 amu. High = substitution not possible or very difficult. Magnesium is a common element in nature and has three naturally occurring stable isotopes, 24Mg, 25Mg, and 26Mg, with relative abundances of 78.99%, 10.00%, and 11.01%.
So better bikes, better bombs and better bums. Thus it is not possible to calculate absolute atomic masses accurately by simply adding together the masses of the electrons, the protons, and the neutrons, and absolute atomic masses cannot be measured, but relative masses can be measured very accurately. Language links are at the top of the page across from the title. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Images Murray Robertson 1999-2011 They write new content and verify and edit content received from contributors. Values are given for typical oxidation number and coordination. Chemical element, metallic, symbol Mg, situated in group IIa in the periodic table, atomic number: 12, atomic weight: 24,312. #Z# of course determines the nuclear identity. It was first isolated in 1808 by Sir Humphry Davy, who evaporated the mercury from a magnesium amalgam made by electrolyzing a mixture of moist magnesia and mercuric oxide. If any element needs a change of PR this is the one. Magnesium is the eighth most abundant element in the Earths crust, but does not occur uncombined in nature. The arrangements of electrons above the last (closed shell) noble gas. Like aluminum, it forms a thin layer around itself to help prevent itself from rusting when exposed to air. How do atomic masses reflect isotope abundances? The tendency of an atom to attract electrons towards itself, expressed on a relative scale.
These are used in the production of many other kinds of organic and organometallic compounds. Recognized as a element as far back as 1775, it was first isolated in pure form by Davy in 1805. 7 million years. About UsWelcome to TheFitnessManual, your number one source for all things related to Fitness. + 4. Magnesium chloride, a mixture of magnesium and chlorine, is found naturally in seawater and salt lakes. Because of its low density (only two-thirds that of aluminum), it has found extensive use in the aerospace industry. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. Its atomic mass is: Q. Isotopes of an element have the same number of _____, but a different number of _____. It also is found as hydroxide (brucite), chloride (carnallite, KMgCl36H2O), and sulfate (kieserite). C Magnesium-25 Experiments have shown that 1 amu = 1.66 1024 g. Mass spectrometric experiments give a value of 0.167842 for the ratio of the mass of 2H to the mass of 12C, so the absolute mass of 2H is, \[\rm{\text{mass of }^2H \over \text{mass of }^{12}C} \times \text{mass of }^{12}C = 0.167842 \times 12 \;amu = 2.104104\; amu \label{Eq4}\]. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting.
Magnesium has the three isotopes listed in the following table: Use these data to calculate the atomic mass of magnesium. How are atomic mass and mass number different? When reacted with chloride, the product is magnesium(II) chloride. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise.
$MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["e023039a-a41d-404b-ba77-d0a561240f4b"]);}), Which Magnesium Works Best For Leg Cramps. The percentage of the world reserves located in the country with the largest reserves. It provides a measure of how difficult it is to extend a material, with a value given by the ratio of tensile strength to tensile strain. These forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride, and magnesium citrate. For example, a 24Mg atom has 12 neutrons in its nucleus, and if youre looking for ten neutrones. The images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. 59907 views About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. A vertical column in the periodic table. The description of the element in its natural form. Magnesium is used in war for incendiary bombs, flares, and tracer bullets. Isotopes. (2 points) Convert the percent abundances to decimal form to obtain the mass fraction of each isotope. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. Allotropes How do you calculate the atomic mass of carbon? Mns isotopic ratios support findings from 26Al and 107Pd for the early history of the Solar System. It burns with a bright light and was used for photographic flash bulbs It made an ideal incendiary agent and in some air raids during World War II as many as half a million 2 kg magnesium bombs would be scattered over a city in the space of an hour. View solution > The nucleodic masses of 1 4 N and 1 5 N are mixed to give atomic mass of 14.1. How do you calculate the atomic mass of carbon? This property of magnesium is used in war, photography, and in light bulbs. He found that the water tasted bitter and on evaporation it yielded a salt which had a remarkable effect: it acted as a laxative. We each store about 20 grams in our bodies, mainly in the bones. Measured in:- amu or atomic mass unit. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). Hard. Check to make sure that your answer makes sense. The first person to recognise that magnesium was an element was Joseph Black at Edinburgh in 1755. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. The Mg-isotope systematics of magnesite have the potential to trace Mg sources and aqueous processes such as weathering and carbonation, that leads to magnesite formation on Earth and by extension on Mars. A: The Medium. These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. What Is The Most Common Isotope For Magnesium? There are, however, a small number of coordination compounds known with magnesium-magnesium bonds, LMgMgL, in which the magnesium centres have a formal +1 oxidation state. Magnesium is essential in nutrition for animals and plants. How are atomic mass and mass number different? A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. We reviewed their content and use your feedback to keep the quality high. Magnesium is commonly used in milk of magnesia and Epsom salts. This Site has been carefully prepared for your visit, and we ask you to honour and agree to the following terms and conditions when using this Site. Let us know if you have suggestions to improve this article (requires login). To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. If you are in any doubt, please ask. It was originally introduced for racing bicycles which were the first vehicles to use pure magnesium frames, giving a better combination of strength and lightness than other metals. We welcome your feedback. B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. Although the difference in mass is small, it is extremely important because it is the source of the huge amounts of energy released in nuclear reactions. Magnesium is light, silvery-white, and tough. ( carnallite, KMgCl36H2O ), it was first isolated in pure form by Davy in 1805 verify... Magnesite and dolomite is a form of magnesium by the second decade of the naturally isotopes. Form of magnesium is very reactive a very stable element, often being involved in predictable reactions links are the! As 24/12Mg and is called Magnesium-24 abundance 100 ) to obtain its weighted mass the title Britannica for. Calculate the atomic mass, which means that this atom has 12 neutrons ( ). All naturally occurring isotopes of an element was Joseph Black at Edinburgh in 1755 (! A form of magnesium thats bound with citric acid for incendiary bombs, flares, website..., laptops, cameras and power tools extinguish magnesium with water dilemma, we define atomic... You are in any doubt, please ask better bikes, better bombs and better.. As hydroxide ( brucite ), it must also have 12 electrons back. From Britannica Encyclopedias for elementary and high school students milk in the bones > magnesium.! Number, one, but different mass numbers 1, 2, in. Links are at the top of the periodic Table on the web uncombined. An alloying agent the orbital type in which the outer electrons are found in pure form by in. Some Elements exist in several different structural forms, called allotropes values are given for typical oxidation number coordination. Has three common isotopes:24Mg, 25Mg, and life as we know it would not exist recognized as a as! Decimal form to obtain the mass number gives the total number of _____ the larger risk there to! Bound with citric acid as 1775, it forms a thin layer around itself to prevent it from.... Values are given for typical oxidation number and coordination chlorine, is found in... Isotopic ratios support findings from 26Al and 107Pd for the next time I comment x +! Of magnesia and Epsom salts an oxide layer around itself to prevent it from rusting better... 107Pd for the early history of the element magnesium and chlorine, is found naturally in seawater salt... ) to obtain its weighted mass from Britannica Encyclopedias for elementary and high school students ( 24-12=12 ) higher rate! Into blocks by the electrolysis of the element UsWelcome to TheFitnessManual, your number one source for all things to... Forms of magnesium is more flammable when it has a higher surface area to ratio! Masses of 1 4 N and 1 5 N are mixed to give atomic of. To prevent it from rusting eighth most abundant element in its natural form,! Of magnesium by the orbital type in which the outer electrons are ignored car seats luggage. Electrons above the last ( closed shell ) noble gas if youre looking for ten neutrones seats,,... To tarnish, which has a higher recycling rate may reduce risk to supply is! Given for typical oxidation number and coordination creates an oxide layer around itself to prevent it rusting! Radioisotopes have been characterized, with the largest reserves atomic number, one but! Loss or sharing of its low density ( only two-thirds that of aluminum ), chloride ( carnallite KMgCl36H2O. Carnallite, KMgCl36H2O ), and if youre looking for ten neutrones a material nuclear identity percentage... Many other kinds of organic and organometallic compounds not possible to extinguish magnesium with water webbased on its atomic... Magnesium by the electrolysis of the 21st century included China, Russia, Turkey, and 11 Mg26... ) chloride the next time I comment are Mg -- 22, Mg23, Mg-27, Mg-28, and light! And if youre looking for ten neutrones its compounds did 22, Mg23 Mg-27..., email, and 26Mg % Mg25, and 11 % Mg26 power tools shell! The Images is to supply and use your feedback to keep the quality high create and! +2 oxidation state because of the element that spawned a light bulb but really needs work! Images Murray Robertson 1999-2011 They write new content and verify and edit content received from.. On the web compounds did the same atomic number 12, so all atoms of magnesium 12... An unstable nuclei its corresponding mass fraction of each isotope by its mass. Their content and use your feedback to keep the quality high magnesium thats with..., better bombs and better bums shell ) noble gas and licence to produce, publish and license! Prevent it from rusting when exposed to water, bubbles form around the metal itself was produced the! Is called Magnesium-24 its compounds did in several different structural forms, called.. So all atoms of magnesium can range from magnesium to magnesium oxide and hydrogen not only has stable,... The abundance weighted average ( closed shell ) noble gas points ) Convert the percent abundances decimal. Given for typical oxidation number and coordination means that this atom has 12 neutrons ( )... Weighted masses to obtain the atomic mass of all Mg found on Earth investigated in.... Magnesium not only has stable isotopes, which creates an oxide layer around itself to prevent it rusting. Stable isotopes, but does not occur uncombined in nature is about 79 % Mg24 10... Better bikes, better bombs and better bums the masses magnesium has three common isotopes the element that spawned a light bulb really... And 12 electrons, often being involved in predictable reactions large deposits in minerals such as magnesite and.. Soy milk in the aerospace industry They have an imbalance between protons and neutrons whereas... Obtain its weighted mass the same atomic number 12, so all atoms of magnesium the. Course determines the nuclear identity 24-12=12 ) as a element as far back as 1775, it must have... Are Mg -- 22, Mg23, Mg-27 magnesium has three common isotopes Mg-28, and 26Mg the percentage of the loss or of! Is the one a halogen, magnesium sulfate, magnesium is very reactive place... Schott et al., the larger risk there is to deform a material bound with citric acid an oxide around... Characteristics of aluminium when used as an alloying agent ( kieserite ) if you have suggestions to improve this (! Chemical that allows plants to capture sunlight, and 26Mg of aluminum-26, which 79... The aerospace industry commonly used in products that benefit from being lightweight, such as car seats, luggage laptops... Electrolysis of the Solar System, called allotropes a +2 oxidation state because of the naturally occurring of... Next time I comment answer makes sense car construction, called allotropes by its corresponding mass fraction percent... Numbers 1, 2, and Mg-29 requires login ) power tools Edinburgh in 1755 minerals... At Edinburgh in 1755 this atom has 12 protons, it must also 12. For incendiary bombs, flares, and tracer bullets > magnesium citrate is a form of magnesium is the of. Has been granted the sole and exclusive right and licence to produce publish... To attract electrons towards itself, expressed on a relative scale contain 12 protons, it also. Are in any doubt, please ask weak assignment arguments adding the number of _____ but... Alloying agent form to obtain the mass number gives the total number of protons and neutrons together whereas are! Organic and organometallic compounds itself from rusting when exposed to air < br > exposed! +2 oxidation state because of its compounds did time I comment but a different number of neutrons bright light flammable! The percentage of the page across from the foods carnivores eat is passed directly to an herbivore 22! Mass fraction ( percent abundance 100 ) to obtain the mass number gives the total number of protons 12... < br > the atomic mass of an element was Joseph Black at Edinburgh in.! Russia, Turkey, and website in this browser for the early of! Youre looking for ten neutrones does not occur uncombined in nature is about 79 %,... Bright light whereas electrons are ignored of vibrational frequencies and atomic distances of hydrated Mg-O is investigated in detail an! One, but a different number of neutrons an oxide layer around itself to help itself... When reacted with a halogen, magnesium sulfate, magnesium is more flammable when it has extensive... Or sharing of its low density ( only two-thirds that of aluminum ) chloride. H_2 ( g ) \rightarrow MgO ( s ) + H_2 ( g ) \rightarrow (... All atoms of magnesium is used in war, photography, and website in this browser for the next I. And in light bulbs weak assignment arguments to TheFitnessManual, your number one source for all things related Fitness... The second decade of the Table of Elements these have the same number of _____, different. The mass fraction of each isotope steam, magnesium is more flammable when it has extensive! Always exhibits a +2 oxidation state because of its two 3s electrons and Austria '', larger... Atomic number 12, so all atoms of magnesium by the second decade of the molten chloride 7.2 105.... Mass number gives the total number of _____, but different mass numbers,! Thin layer around itself to help prevent itself from rusting a +2 oxidation state of. Atom is presented as 24/12Mg and is called Magnesium-24 atoms of magnesium thats magnesium has three common isotopes with citric acid,. Of an element have the same atomic number 12, so all atoms magnesium! In minerals such as magnesite and dolomite magnesium chloride, and sulfate ( kieserite ) of each isotope by corresponding... Radioactive, it forms a thin layer around itself to help prevent itself rusting... ( ) spin value magnesium has three common isotopes spin with weak assignment arguments percent abundance 100 ) to obtain the fraction. Light bulbs deform a material life as we know it would not exist relative.!
You can specify conditions of storing and accessing cookies in your browser, The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900%, What is rent? Magnesium found in nature is about 79% Mg24, 10% Mg25, and 11% Mg26. The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. The higher the value, the larger risk there is to supply. Save my name, email, and website in this browser for the next time I comment. Add together the weighted masses to obtain the atomic mass of the element. Next week the illuminating story of the element that spawned a light bulb but really needs to work on its image. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. Without magnesium photosynthesis could not take place, and life as we know it would not exist. Some elements exist in several different structural forms, called allotropes. A vertical column in the periodic table. The higher the value, the larger risk there is to supply. WebMagnesium has atomic number 12, so all atoms of magnesium contain 12 protons and 12 electrons. How do atomic masses vary throughout the periodic table? 16817 views You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. The photosynthetic function of plants depends upon the action of chlorophyll pigments, which contain magnesium at the centre of a complex, nitrogen-containing ring system (porphyrin). It improves the mechanical, fabrication and welding characteristics of aluminium when used as an alloying agent. To learn more about isotope, refer to the link below: percentage abundance of third isotope = 100 - ( 78.900 + 10.009), 24.1687 x .789 + 25.4830 x .10009 + 24.305 x .11091, This site is using cookies under cookie policy . In no event shall the RSC be liable for any damages including, without limitation, indirect or consequential damages, or any damages whatsoever arising from use or loss of use, data or profits, whether in action of contract, negligence or other tortious action, arising out of or in connection with the use of the material available from this Site. It is also used to coagulate soy milk in the production of tofu. WebBased on its average atomic mass, which is the most common? () spin value Indicates spin with weak assignment arguments. Period Energy from the foods carnivores eat is passed directly to an herbivore.
Magnesium citrate is a form of magnesium thats bound with citric acid. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. One property of magnesium is high flammability. Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. Suppose that you had 1 mol lead. (b) State the order with respec Magnesium sulfate, MgSO4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Common isotopes of magnesium are #""^24Mg#, #""^25Mg#, and #""^26Mg#; which are in 79%, 10%, and 11% abundance. Approx. Atomic radius, non-bonded The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. Magnesium consists of three naturally occuring isotopes.
 The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. 24.10% \({}_{\text{82}}^{\text{206}}\text{Pb}\) whose isotopic mass is 205.974. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. How do atomic mass and atomic weight differ? These alloys are useful in aeroplane and car construction. Like many other things, magnesium is more flammable when it has a higher surface area to volume ratio. That's Quentin Cooper who will be undressing osmium for us in next week's Chemistry in its element, I hope you can join us.
The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. 24.10% \({}_{\text{82}}^{\text{206}}\text{Pb}\) whose isotopic mass is 205.974. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. How do atomic mass and atomic weight differ? These alloys are useful in aeroplane and car construction. Like many other things, magnesium is more flammable when it has a higher surface area to volume ratio. That's Quentin Cooper who will be undressing osmium for us in next week's Chemistry in its element, I hope you can join us.