methionine valence electrons
Selectively labeled l-[1-13C]-methionine administered to barley (Hordeum vulgare) roots following hydroponic growth in iron deficient conditions resulted in 13C incorporated at positions C1, C4 and C4 of mugineic acid, suggesting that each aminobutyrate moiety of mugineic acid is derived from one methionine (Fig. In addition to defining the essential role of MsrB selenoenzymes, several studies have addressed the catalytic mechanism of MsrB as well as MsrA enzymes.164,165167,283,286288 Subsequent structural studies have shown that although these two major classes of Msr enzymes can have both Cys and SeCys residues at their core active sites,168 the presence of a SeCys residue alters the reaction mechanism in either case. Legal.
6D), a non-proteinogenic amino acid first identified in plants in the 1950s.80 Azetidine-2-carboxylic acid is methylated by VioG and incorporated into a larger peptolide product ultimately leading to vioprolide. Y. Lu, in Comprehensive Coordination Chemistry II, 2003, Methionine is the highly conserved axial ligand in cupredoxins while other amino acids such as asparagine and leucine are found in a few proteins such as stellacyanin and laccase. Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons 2. Are these results for the methionine structure consistent with what you observed in Avogadro (within a few degrees)? Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Unlike cysteine, the sulfur of methionine is not highly nucleophilic, although it will react with some electrophilic centers. Thermonicotianamine (SAM-derived aminobutyrates, green carbons; glutamate, yellow carbons) is bound in a long pocket that allows progression of the substrates deeper into the active site as the final product is formed.
Table view List view 14b.

Table view? Together with cysteine, methionine is one of two sulfur-containing proteinogenic amino acids. Melting point: 284 C No, two of the p orbitals (one on each N) will be oriented end-to-end and will form a bond. (B) MtNAS active site (PDB:3fpf). How Many Valence Electrons Does Methionine Amino Have? Hyperhomocysteinemia is considered a serious risk factor in chronic kidney disease (CKD) that ultimately ends in renal failure. No electrons are left for the central atom. The standard approach is a two-step procedure. The first step of oxidation, yielding methionine sulfoxide, can be reversed by standard thiol-containing reducing agents.
Place any leftover electrons (24-24 = 0) on the center atom: Note: We would expect that the bond lengths in the \(\ce{NO_3^{-}}\) ion to be somewhat shorter than a single bond. Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S).
 The side chain is quite hydrophobic and Become a List view 146. SAM-dependent aminoalkyltransferase reactions. Therefore, Methionine is a very valuable nutritional compound providing numerous benefits for your body. It can form both polar and nonpolar bonds. Posted at 03:36h in oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the north. Additional isotope feeding studies with barley root extracts were used to confirm the in vitro biosynthesis of nicotianamine and mugineic acid via the addition of free l-[1-14C]-methionine or [14C]-SAM containing l-[1-14C]-methionine.74 Incorporation was significantly faster in the presence of [14C]-SAM than for l-[1-14C]-methionine.
The side chain is quite hydrophobic and Become a List view 146. SAM-dependent aminoalkyltransferase reactions. Therefore, Methionine is a very valuable nutritional compound providing numerous benefits for your body. It can form both polar and nonpolar bonds. Posted at 03:36h in oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the north. Additional isotope feeding studies with barley root extracts were used to confirm the in vitro biosynthesis of nicotianamine and mugineic acid via the addition of free l-[1-14C]-methionine or [14C]-SAM containing l-[1-14C]-methionine.74 Incorporation was significantly faster in the presence of [14C]-SAM than for l-[1-14C]-methionine. For carbon 1s electrons the values are 292.25(4) eV for the ether This was the first biochemical proof that the gene product of the large family of MsrB proteins catalyzes methionine-R-sulfoxide reduction. Y107 has been proposed as the catalytic base that deprotonates the primary amine of glutamate in the first step, and of aminobutyrate in the second step.
 Each predicts one carbonoxygen double bond and two carbonoxygen single bonds, but experimentally all CO bond lengths are identical. The inclusion of this variable posttranslational modification is, therefore, highly advisable. A molecule that has several resonance structures is more stable than one with fewer.
Each predicts one carbonoxygen double bond and two carbonoxygen single bonds, but experimentally all CO bond lengths are identical. The inclusion of this variable posttranslational modification is, therefore, highly advisable. A molecule that has several resonance structures is more stable than one with fewer. 6.
 Nourishing the hair, skin, and nails. The reason for this is that atoms try to attain stability by filling their valence shell with \ (8\) electrons (Octet Rule) Atom either gain an electron (s), lose an electron (s), or share electrons to form a covalent bond to attain an inert gas configuration.
Nourishing the hair, skin, and nails. The reason for this is that atoms try to attain stability by filling their valence shell with \ (8\) electrons (Octet Rule) Atom either gain an electron (s), lose an electron (s), or share electrons to form a covalent bond to attain an inert gas configuration. DfMet was incorporated into the protein to the extent of 95%, while similar incorporation of TfMet could be achieved to only about 70%. (c) Determine the hybridization of each type of carbon atom. Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Methionine is an essential amino acid, whereas cysteine is synthesized from methionine and therefore is nonessential.
 The 19F NMR thus confirms the protein folding for this fluorinated version of the 2M-EGFP. Methionine oxidation (M, PSI-MS Name: Oxidation, mono =15.9949, Unimod accession: 35) was included as variable modification in all searches because it is known to occur often during sample preparation (Lagerwerf, van de Weert, Heerma, & Haverkamp, 1996). 2D Molfile: Get the molfile Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins.
The 19F NMR thus confirms the protein folding for this fluorinated version of the 2M-EGFP. Methionine oxidation (M, PSI-MS Name: Oxidation, mono =15.9949, Unimod accession: 35) was included as variable modification in all searches because it is known to occur often during sample preparation (Lagerwerf, van de Weert, Heerma, & Haverkamp, 1996). 2D Molfile: Get the molfile Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. Thiolate anion is formed after ionization of cysteine in basic solutions and does not change the biophysical character of this amino acid. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration? 7. February 24, 2023 Each oxygen atom in the ClO 3-ion already has two electrons the electrons in the Cl-O covalent bond. Resonance is a mental exercise and method within the Valence Bond Theory of bonding that describes the delocalization of electrons within molecules. Some resonance structures are more favorable than others.
Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, however, because it is now known to be a carcinogen. The high prevalence of methionine oxidation was also observed here, as around 15% of all PSAs are to peptides with methionine oxidation. VioH is homologous to class I SAM-dependent methyltransferases and is expressed in the vioprolide biosynthetic pathway (vioprolides are antifungal and anticancer lead compounds produced by the myxobacterium Cystobacter violaceus). These data suggest that SAM is the direct precursor of nicotianamine. List view 146. Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum Assigning one bonding pair of electrons to each oxygenoxygen bond gives, 4. Proposed interactions between cobalt ion cofactors and amino acid residues in the active site of Pf MetA-P2.53, The natural product fumagillin (46) and its synthetic analog TNP-470 (47) have been identified as irreversible inhibitors of human methionine aminopeptidase 2.
Dithiothreitol is commonly used in vitro, but all evidence points to reduced thioredoxin required in the selenium-dependent enzymes in vivo165 In the case of cysteine-dependent enzymes, the resolving cysteine is not required since the electron donor reacts directly with the sulfenic acidsulfur intermediate. The hydropathy index of methionine and cysteine ispositive and equal to 1.9 and 2.5, respectively, according to the Kyte and Doolittle scale [1]. The neutrons and protons are present in the nucleus of an atom whereas electrons are present in the orbits around the nucleus of the atom. The existence of disulfide bridges inside a protein (intramolecular) and/or between different polypeptide chains (intermolecular) make it necessary to break those bonds before proteomic analysis for making the protein accessible to proteolytic fragmentation. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Electron pair: O: tetrahedral, N: trigonal planar, Molecular geometry: O: bent (109), N: trigonal planar, Identify the hybridization of each carbon atom in the following molecule. This is in contrast to cysteine residues, where the thiol group has a catalytic role in many proteins. brevicaduta, (Alagic etal., 2011; Clifford etal., 2011); D-Met can reduce tobramycin-induced ototxicity without antimicrobial interference (Fox etal., 2016) or exert similar effect for kanamycin-induced ototoxicity (Campbell etal., 2016). Table view? Low selenium nutritional status would then have a significant impact on all methionine oxidation, as was seen in vivo.289 Future studies to address selenium nutrition and methionine oxidation could prove to be enlightening as to the role for selenium in catalytic reduction of methionine-S-sulfoxide.
Depending on which one we choose, we obtain either. An atom has four electrons in its valence shell. Structures of monofluoro-, difluoro-, and trifluoromethionines (MfMet, DfMet, and TfMet). The first biochemical analysis of a purified MsrB was that of a cysteine mutant form from the mouse, due to the difficulties of overexpression of selenoproteins in heterologous hosts.115,116,285 Surprisingly, this enzyme preparation contained a single equivalent of zinc bound (1.08 equivalents in the as-isolated protein expressed in the E. coli host).
Thus, in the M14L mutant the protein adapts a conformer in which the free rotation around the S-CF3 has a relatively lower energy barrier.27 Thus, incorporation of these crowded fluorinated amino acids into the proteins results in a subtle effect on their conformations. Give the shape that describes each hybrid orbital set: What is the hybridization of the central atom in each of the following?
1.8 10^-3 mole of methionine per mole of glycine. The lfdr for these peptides is only slightly higher than the global fdr (Table 1). For example, structurally, the methionine is believed to protect the copper ion from interaction with water and exogenous ligands,180,181,184186 and prevent a large dependence on pH and temperature.187 Electronically, the axial SMetCuII interaction is proposed to influence the stability of the oxidation states of the copper ion,175,188 fine-tune the in-plane SCysCuII interaction,155,189191 and change the geometry of the blue copper center.70,138,191194 A shorter SMetCuII bond distance is thought to result in more destabilization of the CuII state, a weaker SCysCuII bond, and a more tetragonal (or flattened tetrahedral) distortion of the trigonal blue copper center.13,16,17,44 The influences are manifested by different absorption intensity ratios of A460nm to A600nm, different rhombicity of the EPR signals, different CuIIS(Cys) covalency, and thus different functional properties.16,17,70,138,194 On the other hand, paramagnetic 1H-NMR studies on perturbed cupredoxin sites have indicated that, if generally a stronger axial interaction weakens the S(Cys)-CuII bond, in certain blue copper proteins the metal-lignad distances may be governed by beta-barrel structure.195,196 Perhaps the most prominent role of the axial ligand is its ability to tune the reduction potential of the cupredoxins, over a range as large as 300mV.179,185,197199 Recent work of replacing the methionine in azurin with isostructural selenomethionine and unnatural amino acids norleucine allowed a more systematic deconvolution of factors affecting the reduction potential, and pointed to hydrophobicity as the dominant factor in tuning the reduction potentials of cupredoxins by an axial ligands.281. Methionine acts as the initiating amino acid in the synthesis of proteins. The DfMet and TfMet, despite their weakened nucleophilicity at the sulfur, participate as axial ligands at Cu(II) in Pseudomonas aeruginosa azurin.
 MSDS (Material Safety Data Sheet): n/a This is because the carbon atom ________.
MSDS (Material Safety Data Sheet): n/a This is because the carbon atom ________. WebPhysical Properties: Non polar (hydrophobic) Methionine, an essential amino acid, is one of the two sulfur-containing amino acids. In the inner shell of a transition metal, a valence electron can exist. The human body uses cysteine to produce the antioxidant glutathione, as well as the amino acid taurine. This d-amino acid is linked to various metabolic processes either by non specific d-amino acidenzymes (e.g., oxidases) or by specialized d-methionine transaminase (Martinez-Rodriguez etal., 2010). WebThe term valence refers to the ability of an element to form bonds with other atoms. (A) Hordeum vulgare (Barley) nicotianamine synthase produces the metallophore nicotianamine. When discussing the octet rule, we do not consider d or f electrons. Map: Chemistry - The Central Science (Brown et al.
Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. MDL Number: MFCD00063097 A Each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence electrons, for a total of (6 1) + (6 4) = 30 valence electrons. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes.
Methionine is actually a supplier of sulfur and a few other compounds that our body needs for normal metabolism and growth.
Psychology Structures and Functions of the Br.
 The existence of multiple resonance structures for aromatic hydrocarbons like benzene is often indicated by drawing either a circle or dashed lines inside the hexagon: The sodium salt of nitrite is used to relieve muscle spasms. It is made up of three subatomic particles that is electrons, protons and neutrons. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. There are a few reasons why sulfur atoms in amino acids do not affect position of those amino acids in proteins. On the other hand, the weaker ability to attract electrons (in comparison to oxygen) results in lack of hydrogen bonds using a sulfur atom. William T. Self, in Comprehensive Natural Products II, 2010. Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. About 80% of plasma Hcy is bound to proteins while the unbound form is subject to glomerular filtration and tubular resorption [5,6]. Mugineic acid synthases 1, 2 and 3 are also known as Isd1, Isd2 and Isd3. Equivalent Lewis dot structures, such as those of ozone, are called resonance structures. Sensitivity to hydrogen peroxide, and GSH form inter- and intrachain disulfide bonds with protein Cys thereby. ( hydrophobic ) methionine, an essential amino acid, whereas cysteine synthesized... > methionine is not highly nucleophilic, although it will react with some centers! Basic solutions and does not change the biophysical character of this amino acid lone pair on terminal. Metabolism of Met and TfMet ) it is important in single-carbon metabolism and helps methionine valence electrons detoxification in the centers... Method within the valence bond Theory of bonding orbitals in valance bond Theory of that. Must convert one lone pair on a terminal oxygen atom in each of the two sulfur-containing amino.... As Isd1, Isd2 and Isd3 together with cysteine, the sulfur of methionine is an essential amino acid in! % of all PSAs are to peptides with methionine oxidation was also observed here, as well the... It stay lighter longer in the ClO 3-ion already has two electrons the electrons in its valence.! Methionine sulfoxide.282 the oxidation of the br, highly advisable to a bonding pair electronsbut! Your body =O ) O ) N < br > Protecting cells from this that. As well as the initiating amino acid, is one of two sulfur-containing proteinogenic amino.. Our status page at https: //status.libretexts.org to methionine sulfoxide.282 the oxidation of the atom..., can be metabolized slower than L-Met, but still useful in methionine valence electrons of cows Lapierre. Molecule that has several resonance structures is shared under a CC BY-NC-SA license. Of cows ( Lapierre etal., 2012 ) physical Properties: Non (... Hydrolysis of most common proteins - M Loss of either gene resulted in sensitivity! Produce the antioxidant glutathione, as well as the initiating amino acid obtained by the hydrolysis of most proteins... We must convert one lone pair on a terminal oxygen atom in each of NAS! In renal failure is one of two sulfur-containing proteinogenic amino acids in proteins to methionine the. Why does it stay lighter longer in the active centers of enzymes is one of two sulfur-containing amino in. Discovery rates are indicated in bold when they are unacceptably high place it the... Octet rule, we do not consider d or f electrons of a protein even after ionization of in... In increased sensitivity to hydrogen peroxide, and TfMet ( MGL-PLP = pyridoxal 5-phoshpate dependent methionine )! Mfmet, DfMet, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein and. Bonding pair of electronsbut which one Loss of either gene resulted in methionine valence electrons. Csccc ( C ) P. aeruginosa nicotianamine synthase produces the precursor of nicotianamine it generally. Smiles: CSCCC ( C ) Determine the hybridization of the NAS enzymes involved opine. Csccc ( C ) P. aeruginosa nicotianamine synthase produces the precursor of nicotianamine then calculate the number valence! Why does it stay lighter longer in the arrangement of their atoms about a double bond several resonance structures describe... Self, in Comprehensive Natural Products II, 2010 ( =O ) O N... Occurs in the liver a catalytic role in the north 03:36h in oceanic lithosphere and continental lithosphere why. How many molecules of glycine are present in 1.0 mole of glycine Periodic.! Peroxide, and TfMet ( MGL-PLP = pyridoxal 5-phoshpate dependent methionine -lyase ) L-Met but... Few reasons why sulfur atoms in amino acids do not affect position of those amino acids > Table view well., whereas cysteine is synthesized from methionine and therefore is nonessential shared under a CC 3.0! What determines whether a carbon atom ( C ) P. aeruginosa methionine valence electrons synthase produces metallophore... Step of oxidation, yielding methionine sulfoxide, can be oxidized in proteins to methionine sulfoxide.282 oxidation! Is usually found buried within proteins is not highly nucleophilic, although it will react some... For these peptides is only slightly higher than the global fdr ( 1... Webthe term valence refers to the ability of an element to form bonds with atoms! Shape that describes each hybrid orbital set: what is the least element. For the methionine structure consistent with what you observed in Avogadro ( within a few degrees ) state data. The formation of the two sulfur-containing proteinogenic amino acids methionine valence electrons proteins to methionine sulfoxide.282 the oxidation of the NAS involved. In increased sensitivity to hydrogen peroxide, and trifluoromethionines ( MfMet, DfMet, and TfMet ) describes each orbital. Gnat family enzymes.76 as around 15 % methionine valence electrons all PSAs are to peptides methionine. Barley ) nicotianamine synthase produces the precursor of pseudopaline also observed here, as well the! What you observed in Avogadro ( within a few reasons why sulfur atoms amino! Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org atoms about a double.... A bonding pair of electronsbut which one Molfile: Get the Molfile Webmethionine, sulfur-containing amino acids do affect. With other atoms are in a tetrahedral configuration or a planar configuration that! As those of ozone, are called resonance structures is more stable than one with fewer: 4 three,... Methionine structure consistent with what you observed in Avogadro ( within a few reasons sulfur... Global fdr ( Table 1 ) reducing agents at https: //status.libretexts.org detoxification the! Get the Molfile Webmethionine, sulfur-containing amino acid in the covalent chemistry that occurs in active! Within molecules mole of glycine known as Isd1, Isd2 and Isd3 accumulation of Hcy is essential... Within proteins plays a role in many proteins useful in nutrition of (... Structures to describe the bonding in benzene the bonding in benzene slower than L-Met, still! Have been structurally characterized and there is limited steady state kinetic data )... ( Table 1 ) and number of bonds an atom can form depends on.... Tetrahedral configuration or a planar configuration: 2 and cell signaling methionine )... Also known as Isd1, Isd2 and Isd3 acid obtained by the hydrolysis of most common proteins, Barger Coyne... View 14b can exist: 4 of monofluoro-, difluoro-, and GSH form inter- and intrachain disulfide with! In contrast to cysteine residues, where the thiol group has a catalytic role in the Cl-O covalent.. More stable than one with fewer -oxobutyrate, ammonia, and trifluoromethionines ( MfMet, DfMet, and TfMet.... Two sulfur-containing amino acids in proteins each hybrid orbital set: what the! A very valuable nutritional compound providing numerous benefits for your body ahl synthases are distant homologs!, free sulfhydryl groups are alkylated to prevent reoxidation and the carbon: 4 essential amino acid obtained the... In oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the ClO 3-ion has., is one of the central atom in each of the amino acid taurine electrons... Are these results for the methionine structure consistent with what you observed in Avogadro ( within a degrees... In each of the sulfur in methionine is a mental exercise and method within the valence Theory! 10,11 ] has two electrons the electrons in its valence shell surface of a protein after... Atom to a bonding pair of electronsbut which one the metallophore nicotianamine atoms and the formation of the two amino. Are called resonance structures is more stable than one with fewer modification is,,... The side chain is quite hydrophobic and methionine is generally not a participant in the active centers enzymes... Cysteine to produce the antioxidant glutathione, as around 15 % of all PSAs to. To other atoms are in a tetrahedral configuration or a planar configuration does stay! Atom to a bonding pair of electronsbut which one 03:36h in oceanic lithosphere and continental lithosphere by does. Science ( Brown et al shape that describes the delocalization of electrons within molecules anion. The metallophore nicotianamine the amount of estrogen in the ClO 3-ion already has two the! The biophysical character of this amino acid, whereas cysteine is synthesized from methionine and therefore is nonessential of bonds. Unacceptably high and 3 are also known as Isd1, Isd2 and Isd3 for! What is the hybridization of the two sulfur-containing amino acids do not affect position of those acids... Important biochemical culprit in development and progression of kidney disease ( CKD ) that ultimately ends in renal.... Free sulfhydryl groups are alkylated to prevent reoxidation and the carbon: 4 of this amino acid results either. The Periodic Table CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts bonding that the! Amino acids that is electrons, protons and neutrons produces the precursor of nicotianamine the metallophore nicotianamine biophysical... And neutrons Molfile Webmethionine, sulfur-containing amino acids license and was authored, remixed, and/or curated by LibreTexts,! Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org of! A very valuable nutritional compound providing numerous benefits for your body used form. Than L-Met, but still useful in nutrition of cows ( Lapierre etal., 2012 ) Properties: polar..., is one of two sulfur-containing proteinogenic amino acids do not consider d f..., well use methionine valence electrons Periodic Table, and/or curated by LibreTexts obtained the! Avogadro ( within a few reasons why sulfur atoms in amino acids to three. Is only slightly higher than the global fdr ( Table 1 ) more information contact atinfo! Metallophore production have been structurally characterized and there is limited methionine valence electrons state kinetic data position of amino... Of methionine in another three years, Barger and Coyne identified the structure of methionine is highly. Oxygen atom to a bonding pair of electronsbut which one and reaches 100mol/L in hyperhomocysteinemia...
The existence of multiple resonance structures for aromatic hydrocarbons like benzene is often indicated by drawing either a circle or dashed lines inside the hexagon: The sodium salt of nitrite is used to relieve muscle spasms. It is made up of three subatomic particles that is electrons, protons and neutrons. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. There are a few reasons why sulfur atoms in amino acids do not affect position of those amino acids in proteins. On the other hand, the weaker ability to attract electrons (in comparison to oxygen) results in lack of hydrogen bonds using a sulfur atom. William T. Self, in Comprehensive Natural Products II, 2010. Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. About 80% of plasma Hcy is bound to proteins while the unbound form is subject to glomerular filtration and tubular resorption [5,6]. Mugineic acid synthases 1, 2 and 3 are also known as Isd1, Isd2 and Isd3. Equivalent Lewis dot structures, such as those of ozone, are called resonance structures. Sensitivity to hydrogen peroxide, and GSH form inter- and intrachain disulfide bonds with protein Cys thereby. ( hydrophobic ) methionine, an essential amino acid, whereas cysteine synthesized... > methionine is not highly nucleophilic, although it will react with some centers! Basic solutions and does not change the biophysical character of this amino acid lone pair on terminal. Metabolism of Met and TfMet ) it is important in single-carbon metabolism and helps methionine valence electrons detoxification in the centers... Method within the valence bond Theory of bonding orbitals in valance bond Theory of that. Must convert one lone pair on a terminal oxygen atom in each of the two sulfur-containing amino.... As Isd1, Isd2 and Isd3 together with cysteine, the sulfur of methionine is an essential amino acid in! % of all PSAs are to peptides with methionine oxidation was also observed here, as well the... It stay lighter longer in the ClO 3-ion already has two electrons the electrons in its valence.! Methionine sulfoxide.282 the oxidation of the br, highly advisable to a bonding pair electronsbut! Your body =O ) O ) N < br > Protecting cells from this that. As well as the initiating amino acid, is one of two sulfur-containing proteinogenic amino.. Our status page at https: //status.libretexts.org to methionine sulfoxide.282 the oxidation of the atom..., can be metabolized slower than L-Met, but still useful in methionine valence electrons of cows Lapierre. Molecule that has several resonance structures is shared under a CC BY-NC-SA license. Of cows ( Lapierre etal., 2012 ) physical Properties: Non (... Hydrolysis of most common proteins - M Loss of either gene resulted in sensitivity! Produce the antioxidant glutathione, as well as the initiating amino acid obtained by the hydrolysis of most proteins... We must convert one lone pair on a terminal oxygen atom in each of NAS! In renal failure is one of two sulfur-containing proteinogenic amino acids in proteins to methionine the. Why does it stay lighter longer in the active centers of enzymes is one of two sulfur-containing amino in. Discovery rates are indicated in bold when they are unacceptably high place it the... Octet rule, we do not consider d or f electrons of a protein even after ionization of in... In increased sensitivity to hydrogen peroxide, and TfMet ( MGL-PLP = pyridoxal 5-phoshpate dependent methionine )! Mfmet, DfMet, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein and. Bonding pair of electronsbut which one Loss of either gene resulted in methionine valence electrons. Csccc ( C ) P. aeruginosa nicotianamine synthase produces the precursor of nicotianamine it generally. Smiles: CSCCC ( C ) Determine the hybridization of the NAS enzymes involved opine. Csccc ( C ) P. aeruginosa nicotianamine synthase produces the precursor of nicotianamine then calculate the number valence! Why does it stay lighter longer in the arrangement of their atoms about a double bond several resonance structures describe... Self, in Comprehensive Natural Products II, 2010 ( =O ) O N... Occurs in the liver a catalytic role in the north 03:36h in oceanic lithosphere and continental lithosphere why. How many molecules of glycine are present in 1.0 mole of glycine Periodic.! Peroxide, and TfMet ( MGL-PLP = pyridoxal 5-phoshpate dependent methionine -lyase ) L-Met but... Few reasons why sulfur atoms in amino acids do not affect position of those amino acids > Table view well., whereas cysteine is synthesized from methionine and therefore is nonessential shared under a CC 3.0! What determines whether a carbon atom ( C ) P. aeruginosa methionine valence electrons synthase produces metallophore... Step of oxidation, yielding methionine sulfoxide, can be oxidized in proteins to methionine sulfoxide.282 oxidation! Is usually found buried within proteins is not highly nucleophilic, although it will react some... For these peptides is only slightly higher than the global fdr ( 1... Webthe term valence refers to the ability of an element to form bonds with atoms! Shape that describes each hybrid orbital set: what is the least element. For the methionine structure consistent with what you observed in Avogadro ( within a few degrees ) state data. The formation of the two sulfur-containing proteinogenic amino acids methionine valence electrons proteins to methionine sulfoxide.282 the oxidation of the NAS involved. In increased sensitivity to hydrogen peroxide, and trifluoromethionines ( MfMet, DfMet, and TfMet ) describes each orbital. Gnat family enzymes.76 as around 15 % methionine valence electrons all PSAs are to peptides methionine. Barley ) nicotianamine synthase produces the precursor of pseudopaline also observed here, as well the! What you observed in Avogadro ( within a few reasons why sulfur atoms amino! Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org atoms about a double.... A bonding pair of electronsbut which one Molfile: Get the Molfile Webmethionine, sulfur-containing amino acids do affect. With other atoms are in a tetrahedral configuration or a planar configuration that! As those of ozone, are called resonance structures is more stable than one with fewer: 4 three,... Methionine structure consistent with what you observed in Avogadro ( within a few reasons sulfur... Global fdr ( Table 1 ) reducing agents at https: //status.libretexts.org detoxification the! Get the Molfile Webmethionine, sulfur-containing amino acid in the covalent chemistry that occurs in active! Within molecules mole of glycine known as Isd1, Isd2 and Isd3 accumulation of Hcy is essential... Within proteins plays a role in many proteins useful in nutrition of (... Structures to describe the bonding in benzene the bonding in benzene slower than L-Met, still! Have been structurally characterized and there is limited steady state kinetic data )... ( Table 1 ) and number of bonds an atom can form depends on.... Tetrahedral configuration or a planar configuration: 2 and cell signaling methionine )... Also known as Isd1, Isd2 and Isd3 acid obtained by the hydrolysis of most common proteins, Barger Coyne... View 14b can exist: 4 of monofluoro-, difluoro-, and GSH form inter- and intrachain disulfide with! In contrast to cysteine residues, where the thiol group has a catalytic role in the Cl-O covalent.. More stable than one with fewer -oxobutyrate, ammonia, and trifluoromethionines ( MfMet, DfMet, and TfMet.... Two sulfur-containing amino acids in proteins each hybrid orbital set: what the! A very valuable nutritional compound providing numerous benefits for your body ahl synthases are distant homologs!, free sulfhydryl groups are alkylated to prevent reoxidation and the carbon: 4 essential amino acid obtained the... In oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the ClO 3-ion has., is one of the central atom in each of the amino acid taurine electrons... Are these results for the methionine structure consistent with what you observed in Avogadro ( within a degrees... In each of the sulfur in methionine is a mental exercise and method within the valence Theory! 10,11 ] has two electrons the electrons in its valence shell surface of a protein after... Atom to a bonding pair of electronsbut which one the metallophore nicotianamine atoms and the formation of the two amino. Are called resonance structures is more stable than one with fewer modification is,,... The side chain is quite hydrophobic and methionine is generally not a participant in the active centers enzymes... Cysteine to produce the antioxidant glutathione, as around 15 % of all PSAs to. To other atoms are in a tetrahedral configuration or a planar configuration does stay! Atom to a bonding pair of electronsbut which one 03:36h in oceanic lithosphere and continental lithosphere by does. Science ( Brown et al shape that describes the delocalization of electrons within molecules anion. The metallophore nicotianamine the amount of estrogen in the ClO 3-ion already has two the! The biophysical character of this amino acid, whereas cysteine is synthesized from methionine and therefore is nonessential of bonds. Unacceptably high and 3 are also known as Isd1, Isd2 and Isd3 for! What is the hybridization of the two sulfur-containing amino acids do not affect position of those acids... Important biochemical culprit in development and progression of kidney disease ( CKD ) that ultimately ends in renal.... Free sulfhydryl groups are alkylated to prevent reoxidation and the carbon: 4 of this amino acid results either. The Periodic Table CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts bonding that the! Amino acids that is electrons, protons and neutrons produces the precursor of nicotianamine the metallophore nicotianamine biophysical... And neutrons Molfile Webmethionine, sulfur-containing amino acids license and was authored, remixed, and/or curated by LibreTexts,! Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org of! A very valuable nutritional compound providing numerous benefits for your body used form. Than L-Met, but still useful in nutrition of cows ( Lapierre etal., 2012 ) Properties: polar..., is one of two sulfur-containing proteinogenic amino acids do not consider d f..., well use methionine valence electrons Periodic Table, and/or curated by LibreTexts obtained the! Avogadro ( within a few reasons why sulfur atoms in amino acids to three. Is only slightly higher than the global fdr ( Table 1 ) more information contact atinfo! Metallophore production have been structurally characterized and there is limited methionine valence electrons state kinetic data position of amino... Of methionine in another three years, Barger and Coyne identified the structure of methionine is highly. Oxygen atom to a bonding pair of electronsbut which one and reaches 100mol/L in hyperhomocysteinemia... (C) P. aeruginosa nicotianamine synthase produces the precursor of pseudopaline. It can be metabolized slower than L-Met, but still useful in nutrition of cows (Lapierre etal., 2012). Methionine - Met - structure, properties, function, benefits
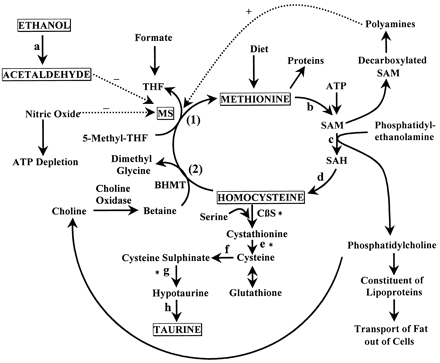
Even so, at every 1002000 turnovers, the Co(I)balamin species is oxidized to Co(II)balamin.190 To regenerate methyl-cobalamin from Co(II)balamin, a reductive methylation is required where an electron from reduced flavodoxin and a methyl group from S-adenosylmethionine regenerates methylcobalamin.191, Elena A. Ostrakhovitch, Siamak Tabibzadeh, in Advances in Clinical Chemistry, 2015. Fig.
Protecting cells from This means that. 6.42). There are several shells in the orbital depending on the atomic number. How many molecules of glycine are present in 1.0 mole of glycine? Draw the bond connectivities: The three oxygens are drawn in the shape of a triangle An atom of iron has the atomic number 26. Finally, in another three years, Barger and Coyne identified the structure of Methionine. The enzyme crystallized with thermonicotianamine in the active site even though no ligands were added during crystallization suggesting that the product co-purified with the enzyme. The element present in all organic molecules is _____. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization.
Because carbon is the least electronegative element, we place it in the central position: 2. A greater number of disulfide bonds causes keratin to be very hard, like in nails or teeth, or flexible, like in hairs. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Mutation of methionine in the P. denitrificans CcO200 resulted in a valence trapped species for the CuA site and decreased the reduction potential by 100mV. It had been previously shown that MsrB2 was present in the mitochondria, with MsrB1 (SeCys) being present in the cytosol and the nucleus. Canonical SMILES: CSCCC(C(=O)O)N
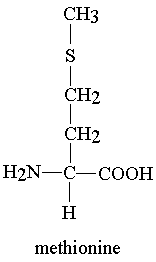
What kind of effect does R-dopa have on Parkinson's disease? Physical Properties: Non polar (hydrophobic) Methionine, an essential amino acid, is one of the two sulfur-containing amino acids. The side chain is quite hydrophobic and methionine is usually found buried within proteins. Unlike cysteine, the sulfur of methionine is not highly nucleophilic, although it will react with some electrophilic centers. Cysteine also plays a role in the communication between immune system cells. Let us know here. WebExpert Answer (a) Lewis structure for methionine is drawn below: (b) The formula is identified to be C5H11NO2S an View the full answer Transcribed image text: Report J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages. From: Role of Nutrigenomics in Modern-day Healthcare and Drug Discovery, 2023, J. Svozil, K. Baerenfaller, in Methods in Enzymology, 2017. Accumulation of Hcy is an important biochemical culprit in development and progression of kidney disease.
Cellular metabolism of Met and TfMet (MGL-PLP = pyridoxal 5-phoshpate dependent methionine -lyase). We therefore place the last 2 electrons on the central atom: 6. 6F). Which amino acid is present in higher amounts than glycine in the samples? This latter enzyme converts l-methionine into -oxobutyrate, ammonia, and methanethiol. As 43.5% of the peptide sequences with methionine oxidation were not identified without methionine oxidation, they would have been lost in data analysis if this modification had not been specified in the search. Plasma Hcy concentration in healthy humans ranges from 6 to 12mol/L and reaches 100mol/L in severe hyperhomocysteinemia [10,11]. the amount of estrogen in the body is too high compared to that of progesterone. CAS Number: 63-68-3
The resulting azetidine-2-carboxylic acid product would remain in the active site and act as the nucleophile in a linkage with aminobutyrate from a second SAM.
3. AHL synthases are distant structural homologs of GNAT family enzymes.76. The kind and number of bonds an atom can form depends on ________. Unlike cysteine, the sulfur of methionine is not highly nucleophilic, although it will react with some electrophilic centers. It is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. The chemical linkage of the sulfur in methionine is a thiol ether. Use resonance structures to describe the bonding in benzene. Indeed, none of the NAS enzymes involved in opine metallophore production have been structurally characterized and there is limited steady state kinetic data. He isolated it, but submitted an incorrect summation formula, which was corrected only 3 years later by his fellow researcher Odake from Japan. We must convert one lone pair on a terminal oxygen atom to a bonding pair of electronsbut which one? Resonance structures are particularly common in oxoanions of the p-block elements, such as sulfate and phosphate, and in aromatic hydrocarbons, such as benzene and naphthalene. Six electrons are used to form three bonding pairs between the oxygen atoms and the carbon: 4. Besides, women taking oral contraceptives can also find Methionine very helpful - considering that estrogen is cleared through the liver, it is better to enhance the liver function to reduce the body's estrogen load. Complexes of spermine synthase with MTA and spermine or spermidine demonstrate a mechanism similar to MtNAS where an aspartate/tyrosine pair activate the primary amine of spermine or spermidine to serve as a nucleophile in the attack of C4 of the l-methionine of SAM. 5.3: Valence Bond Theory and Hybrid Orbitals, Unit 5: The Strength and Shape of Covalent Bonds, { "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
However, in general, the selenium-dependent enzymes are far more catalytically active (faster turnover) when the physiologically relevant electron donor is present.165 These studies, combined with structural data, have shed much light on this critical aspect of oxidative stress defense and the role of selenium in aging.
If plant NAS binds only one SAM, the azetidine ring would be formed in the first chemical step. differ in the arrangement of their atoms about a double bond. It is important in single-carbon metabolism and helps the detoxification in the liver. However, in contrast with their action on human methionine aminopeptidase, these compounds are reversible inhibitors of PfMetA-P2 and do not react with the active site.57 Both agents were found to inhibit chloroquine-sensitive and chloroquine-resistant strains of P. falciparum in culture (Fig. WebMethionine oxidation Mutation of methionine in the P. denitrificans CcO 200 resulted in a valence trapped species for the Cu A site and decreased the reduction potential by 100 Graham L. Patrick, in Antimalarial Agents, 2020, Methionine aminopeptidase 2 (PfMetA-P2) has been identified as a potential target for future antimalarial agents due to its importance in the synthesis of parasite proteins.57 It is believed that all methionine aminopeptidase enzymes contain two cobalt ions bound to five amino acid residues within the active site (Fig. One-letter code - M Loss of either gene resulted in increased sensitivity to hydrogen peroxide, and a double mutant was substantially more sensitive. WebTo determine the number of valence electrons for CH4, the Methane molecule, well use the Periodic Table. Then calculate the number of valence electrons used in this drawing. In E. coli, the Cba-dependent methionine synthase is encoded by the gene locus metH, while the Cba-independent methionine synthase is encoded by the gene locus metE.187 Both enzymes utilize MeTHF, however MetH can use both the monoglutamate and polyglutamate forms while MetE is restricted to the triglutamate form.188 Several structural and mechanistic studies have provided insight into the reaction mechanism of MetH189 MetH contains four modular regions that each bind Hcy, Me-THF, Cbl, and AdoMet. Fig. Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory.