olivine crystal structure
These phase transitions lead to a discontinuous increase in the density of the Earth's mantle that can be observed by seismic methods. Both have model formulas of XY 2 O 4 (i.e., one tetrahedral site and two octahedral sites). Teach the Earth the portal for Earth Education, From NAGT's On the Cutting Edge Collection, This activity was selected for the On the Cutting Edge Reviewed Teaching Collection, This activity has received positive reviews in a peer review process involving five review categories. This magma then crystallizes to those mafic rocks that include gabbro, basalt, and others. Olivine is classified as a nesosilicate which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds from interstitial cations. The olivine structure contains two cation positions - usualy labeled as M1 and M2 - which are not exactly the same. Olivine mineral occurs in both the magic as well as ultramafic igneous olivine rock. The material has attracted attention as a component of lithium iron phosphate batteries,[1] a type of Li-ion battery. If you are using CrystalMaker, you can perform this operation by increasing the plot range of the model by clicking on Transform -> Set Range and increasing the maximum range for the x-axis to 1.8 angstroms. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. In patent lawsuits in the US in 2005 and 2006, the University of Texas at Austin|University of Texas-Austin and Hydro-Qubec claimed that LiFePO4 as the cathode infringed their patents, .mw-parser-output .citation{word-wrap:break-word}.mw-parser-output .citation:target{background-color:rgba(0,127,255,0.133)}US 5910382 and US 6514640. Get a Britannica Premium subscription and gain access to exclusive content. Gem-quality forsterite olivine is known as peridot. It is of the common minerals that are available in most common across all the world. It is known as enhanced weathering. WebCrystal structures of Olivine group - A 3D model collection by Museum of Mineralogy and Petrography, UAIC (@MineralogyPetrographyMuseum) - Sketchfab. As of 2020, an organization named LifePO+C claims to own the key IP and offers licenses. Most of the naturally occurring olivines are intermediate in composition to these two end-members and have the general formula (Mg, Fe)2SiO4. Are they connected to each other? Conductive carbon is obtained by adding polyethylene glycol to the solution followed by thermal processing. If you are looking for olivine meaning, then it is a complex silicate that includes magnesium as well as iron that is used in refractories. Minerals with independent tetrahedral structures are called neosilicates (or orthosilicates). where X = Mg ,Fe2+, or Ca; Y = Al, Cr, or Fe3+; We support geoscience education at every level. This arises from the SiO. Manganese, phosphate, iron, and lithium also form an olivine structure. The primary component of the Earth's upper mantle,[9] it is a common mineral in Earth's subsurface, but weathers quickly on the surface.
In the olivine structure, rigid tetrahedral edges and shared octahedral edges form columns of corner-sharing trigonal dipyramids parallel to the a axis. The root patents of LFP compounds are held by four organizations. It has an orthorhombic crystal system. For viewing with XtalDraw ( more info) , Show details and options. The most notable difference between lithium iron phosphate and lead acid is the fact that the lithium battery capacity shows only a small dependence on the discharge rate. Soc., Volume 144, Issue 4, pp. Click on the OLIVINE file in the XtalDraw folder. This solution includes forsterite and fayalite. This structure is a useful contributor to the cathode of lithium rechargeable batteries. However, the O3 is present in the general position. In addition to olivine, other common neosilicate minerals include garnet, topaz, kyanite, and zircon. The aggregated lithium ions are deposited on the surface of electrodes in the form of plates or even dendrites which may penetrate the separators, short-circuiting the battery completely.[37].

 Most olivine is used in metallurgical strategies as a slag conditioner. The name Olivine is introduced because of its usual olive-green color element. The end-products of these reactions are Silicon Dioxide, small quantities of iron oxide, as well as magnesium carbonate. The initial discharge capacities for LFP/C samples at temperatures of 23, 0, -10, and -20C are 141.8, 92.7, 57.9 and 46.7mAh/g with coulombic efficiency 91.2%, 74.5%, 63.6% and 61.3%. [27] LiMPO4 with 1 wt% of metal additives has a reversible capacity up to 140mAh/g and better efficiency under high discharge current. However, the O, Ans: Olivine is used across many places around the world. WebThe crystal structure of olivine is built from independent silica tetrahedra. 1188-1194 (April 1997), Malik, R.; Abdellahi, A.; Ceder, G., "A Critical Review of the Li Insertion Mechanisms in, Claim Construction; Insights from the Lithium Battery Patent Infringement Case." WebThe crystal structure of olivine is built from independent silica tetrahedra. After a Markman hearing, on April 27, 2011, the Western District Court of Texas held that the claims of the reexamined patents had a narrower scope than as originally granted. Although it depends on the sizes of the grains; however, the process is quite faster. Upon removal of Li, the material converts to the ferric form FePO4.[14]. [15] Olivine and high pressure structural variants constitute over 50% of the Earth's upper mantle, and olivine is one of the Earth's most common minerals by volume. Gem-quality olivine is used as a gemstone called peridot. Many related routes have been described including those that use hydrothermal synthesis. Where does Olivine Do in Extra-Terrestrial Regions? Order/Disorder Within Crystal Structures as a Function of Temperature, http://serc.carleton.edu/research_education/crystallography/olivine/index.html, Teaching Symmetry Using Kinesthetic LearningAn Exercise Using "Old Time" Dances, Minerals, Inclusions and Volcanic Processes, Impacts of Resource Development on Native American Lands, Registry of Analytical Geochemical Equipment, All Mineralogy related materials from across Teach the Earth, Schedule of Upcoming Workshops and Webinars, May 2023 Workshop, Advancing FEW-Nexus-based Education through Research, an article in The Chronicle For Higher Education, Submit abstracts (for oral, poster, teaching demo, and Share-a-Thon sessions) by, Receive early registration discount - Register by, Alignment of Learning Goals, Activities, and Assessments, Robustness (usability and dependability of all components), Completeness of the ActivitySheet web page, students should be familiar with the olivine crystal structure, students should be familiar with the concept of order/disorder within crystals, students should know how to use Excel to make x-y graphs, this activity could supplement class lectures on order/disorder, this activity could supplement class lectures on solid solutions, this activity could supplement class lectures on solid solutions nesosilicate mineral structures, students should be able to use the American Mineralogist Crystal Structure Database to obtain crystal structures of synthetic olivines, students should be able to use a crystallographic visualization program (such as CrystalMaker or Xtaldraw) to identify which elements are housed in which crystallographic sites in the olivine structure, students should be able to use downloaded olivine compositions and Excel to determine the ordering of M1 and M2 cations within olivine, application of the abstract concept of crystallographic order to a dataset in which the amount of order within two cation sites is temperature-dependent, recognition of the relationship between crystallographic order and entropy, using crystallographic software to solve geologic problems. The characteristic red color is reflected in several local names with "red" such as Raudbergvik (Red rock bay) or Raudnakken (Red ridge). Your membership is helping to ensure that this site can continue to serve geoscience educators. Olivine is an abundant mineral in most mafic igneous rocks and the most abundant mineral in the earths upper mantle. [13], In LiFePO4, lithium has a +1 charge, iron +2 charge balancing the 3 charge for phosphate. [19] These patents underlie mature mass production technologies. The Olivine is a mineral that is basically iron silicon blended with magnesium.
Most olivine is used in metallurgical strategies as a slag conditioner. The name Olivine is introduced because of its usual olive-green color element. The end-products of these reactions are Silicon Dioxide, small quantities of iron oxide, as well as magnesium carbonate. The initial discharge capacities for LFP/C samples at temperatures of 23, 0, -10, and -20C are 141.8, 92.7, 57.9 and 46.7mAh/g with coulombic efficiency 91.2%, 74.5%, 63.6% and 61.3%. [27] LiMPO4 with 1 wt% of metal additives has a reversible capacity up to 140mAh/g and better efficiency under high discharge current. However, the O, Ans: Olivine is used across many places around the world. WebThe crystal structure of olivine is built from independent silica tetrahedra. 1188-1194 (April 1997), Malik, R.; Abdellahi, A.; Ceder, G., "A Critical Review of the Li Insertion Mechanisms in, Claim Construction; Insights from the Lithium Battery Patent Infringement Case." WebThe crystal structure of olivine is built from independent silica tetrahedra. After a Markman hearing, on April 27, 2011, the Western District Court of Texas held that the claims of the reexamined patents had a narrower scope than as originally granted. Although it depends on the sizes of the grains; however, the process is quite faster. Upon removal of Li, the material converts to the ferric form FePO4.[14]. [15] Olivine and high pressure structural variants constitute over 50% of the Earth's upper mantle, and olivine is one of the Earth's most common minerals by volume. Gem-quality olivine is used as a gemstone called peridot. Many related routes have been described including those that use hydrothermal synthesis. Where does Olivine Do in Extra-Terrestrial Regions? Order/Disorder Within Crystal Structures as a Function of Temperature, http://serc.carleton.edu/research_education/crystallography/olivine/index.html, Teaching Symmetry Using Kinesthetic LearningAn Exercise Using "Old Time" Dances, Minerals, Inclusions and Volcanic Processes, Impacts of Resource Development on Native American Lands, Registry of Analytical Geochemical Equipment, All Mineralogy related materials from across Teach the Earth, Schedule of Upcoming Workshops and Webinars, May 2023 Workshop, Advancing FEW-Nexus-based Education through Research, an article in The Chronicle For Higher Education, Submit abstracts (for oral, poster, teaching demo, and Share-a-Thon sessions) by, Receive early registration discount - Register by, Alignment of Learning Goals, Activities, and Assessments, Robustness (usability and dependability of all components), Completeness of the ActivitySheet web page, students should be familiar with the olivine crystal structure, students should be familiar with the concept of order/disorder within crystals, students should know how to use Excel to make x-y graphs, this activity could supplement class lectures on order/disorder, this activity could supplement class lectures on solid solutions, this activity could supplement class lectures on solid solutions nesosilicate mineral structures, students should be able to use the American Mineralogist Crystal Structure Database to obtain crystal structures of synthetic olivines, students should be able to use a crystallographic visualization program (such as CrystalMaker or Xtaldraw) to identify which elements are housed in which crystallographic sites in the olivine structure, students should be able to use downloaded olivine compositions and Excel to determine the ordering of M1 and M2 cations within olivine, application of the abstract concept of crystallographic order to a dataset in which the amount of order within two cation sites is temperature-dependent, recognition of the relationship between crystallographic order and entropy, using crystallographic software to solve geologic problems. The characteristic red color is reflected in several local names with "red" such as Raudbergvik (Red rock bay) or Raudnakken (Red ridge). Your membership is helping to ensure that this site can continue to serve geoscience educators. Olivine is an abundant mineral in most mafic igneous rocks and the most abundant mineral in the earths upper mantle. [13], In LiFePO4, lithium has a +1 charge, iron +2 charge balancing the 3 charge for phosphate. [19] These patents underlie mature mass production technologies. The Olivine is a mineral that is basically iron silicon blended with magnesium. Sci. Many finest Olivine is obtained from mantle rocks that are present in the Red Sea. We are excited to announce Rory McFadden will be joining SERC this June as a new Science Education Associate. Adding conducting particles in delithiated FePO4 raises its electron conductivity. WebComparisons of structural features of olivine ( phase), spinel ( phase), and the modified spinel ( phase) lead to predictions of possible mechanisms for the olivine spinel transitions. With general chemical formula of LiMPO4, compounds in the LiFePO4 family adopt the olivine structure.
 Some of the finest gem-quality olivine has been obtained from a body of mantle rocks on Zabargad Island in the Red Sea.[12][13]. WebThe crystal structure of olivine is built from independent silica tetrahedra. In the olivine structure, rigid tetrahedral edges and shared octahedral edges form columns of corner-sharing trigonal dipyramids parallel to the a axis. Coauthor of. Less water means less gas (steam) to vent from the mold as metal is poured into the mold. WebComparisons of structural features of olivine ( phase), spinel ( phase), and the modified spinel ( phase) lead to predictions of possible mechanisms for the olivine spinel transitions. [30] Vapor phase deposition produces a thin film LiMPO4. The O, is present on the inversion center. WebOlivine usually only occurs as part of a rock mass, but when it does form recognizable crystals these are transparent to translucent, olive-green crystals that typically have a granular shape. Olivines are an important rock-forming mineral group. This structure is a useful contributor to the cathode of lithium rechargeable batteries. [35] Another possible cause of the lowered capacity formation is lithium plating. Olivine mineral occurs in both the magic as well as ultramafic igneous olivine rock. (Silica) units joined by many divalent cations of metals with oxygen in the silica that is again bound to just three ions of metals. The olivine chemical formula is (Mg2+Fe2+)2SiO4. Moreover, because olivine is so abundant, more water may be dissolved in olivine of the mantle than is contained in Earth's oceans. Minerals in the olivine group crystallize in the orthorhombic system (space group Pbnm) with isolated silicate tetrahedra, meaning that olivine is a nesosilicate. Fe-rich olivine content can only be present if there are stable quantities of quartz or tridymite available. The phosphate groups, PO4, are tetrahedral.
Some of the finest gem-quality olivine has been obtained from a body of mantle rocks on Zabargad Island in the Red Sea.[12][13]. WebThe crystal structure of olivine is built from independent silica tetrahedra. In the olivine structure, rigid tetrahedral edges and shared octahedral edges form columns of corner-sharing trigonal dipyramids parallel to the a axis. Coauthor of. Less water means less gas (steam) to vent from the mold as metal is poured into the mold. WebComparisons of structural features of olivine ( phase), spinel ( phase), and the modified spinel ( phase) lead to predictions of possible mechanisms for the olivine spinel transitions. [30] Vapor phase deposition produces a thin film LiMPO4. The O, is present on the inversion center. WebOlivine usually only occurs as part of a rock mass, but when it does form recognizable crystals these are transparent to translucent, olive-green crystals that typically have a granular shape. Olivines are an important rock-forming mineral group. This structure is a useful contributor to the cathode of lithium rechargeable batteries. [35] Another possible cause of the lowered capacity formation is lithium plating. Olivine mineral occurs in both the magic as well as ultramafic igneous olivine rock. (Silica) units joined by many divalent cations of metals with oxygen in the silica that is again bound to just three ions of metals. The olivine chemical formula is (Mg2+Fe2+)2SiO4. Moreover, because olivine is so abundant, more water may be dissolved in olivine of the mantle than is contained in Earth's oceans. Minerals in the olivine group crystallize in the orthorhombic system (space group Pbnm) with isolated silicate tetrahedra, meaning that olivine is a nesosilicate. Fe-rich olivine content can only be present if there are stable quantities of quartz or tridymite available. The phosphate groups, PO4, are tetrahedral. 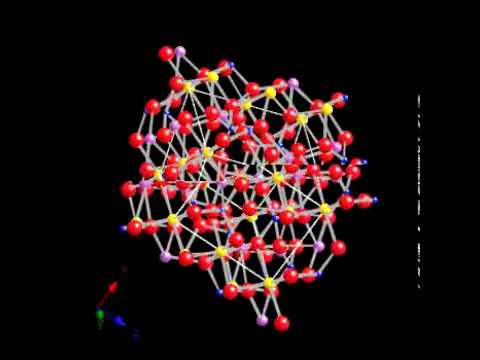 Most olivine is used in metallurgical processes as a slag conditioner. The authors of this study synthesized Fa50 olivine olivine (MgFeSiO4) in an experimental apparatus at temperatures ranging from 100 to 1250 C, quenched the experiments, and used in situ neutron powder diffraction techniques to investigate changes in the synthesized olivines as a function of temperature. Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Forsterite's melting temperature is unusually high at atmospheric pressure, almost 1,900C (3,450F), while fayalite's is much lower about 1,200C (2,190F). Compare the olivine structure to the spinel structure. Also, many people call it chrysolite, which means gold and stone in Greek. The most abundant olivines occur in the system from forsterite (Mg2SiO4) to fayalite (Fe2SiO4). [17], Olivine pine forest (a plant community) is unique to Norway. The X-site is 8 coordinated, the Y -site is 6-coordinated [4] As the first commercial LiMPO4 was C/LiFePO4, the whole group of LiMPO4 is informally called lithium iron phosphate or LiFePO4. This mineralogical association is diagnostic of the relatively high temperatures of crystallization of mafic rock types. Open the Crystal Structures Library on the CrystalMaker disc, and click on. End members are forsterite (Mg2SiO 4) and fayalite (Fe 2+2 SiO 4 ). Strm wrote that in Norddal district large quantities of olivine were broken from the bedrock and used as sharpening stones. Traditional LiCoO2 with oxide coating shows improved cycling performance. It also has spinel-like elements that are equivalent to magnetite; however, it just uses a single quadrivalent and a couple of divalent cations.
Most olivine is used in metallurgical processes as a slag conditioner. The authors of this study synthesized Fa50 olivine olivine (MgFeSiO4) in an experimental apparatus at temperatures ranging from 100 to 1250 C, quenched the experiments, and used in situ neutron powder diffraction techniques to investigate changes in the synthesized olivines as a function of temperature. Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna. Forsterite's melting temperature is unusually high at atmospheric pressure, almost 1,900C (3,450F), while fayalite's is much lower about 1,200C (2,190F). Compare the olivine structure to the spinel structure. Also, many people call it chrysolite, which means gold and stone in Greek. The most abundant olivines occur in the system from forsterite (Mg2SiO4) to fayalite (Fe2SiO4). [17], Olivine pine forest (a plant community) is unique to Norway. The X-site is 8 coordinated, the Y -site is 6-coordinated [4] As the first commercial LiMPO4 was C/LiFePO4, the whole group of LiMPO4 is informally called lithium iron phosphate or LiFePO4. This mineralogical association is diagnostic of the relatively high temperatures of crystallization of mafic rock types. Open the Crystal Structures Library on the CrystalMaker disc, and click on. End members are forsterite (Mg2SiO 4) and fayalite (Fe 2+2 SiO 4 ). Strm wrote that in Norddal district large quantities of olivine were broken from the bedrock and used as sharpening stones. Traditional LiCoO2 with oxide coating shows improved cycling performance. It also has spinel-like elements that are equivalent to magnetite; however, it just uses a single quadrivalent and a couple of divalent cations.  There are two symmetrically nonequivalent octahedral sites, M1 and M2. Minerals in the Olivine Series are quite hard, falling from 6.5 to 7.0 on the Mohs hardness scale. It also has spinel-like elements that are equivalent to magnetite; however, it just uses a single quadrivalent and a couple of divalent cations. The students are instructed on how to manipulate the structures and are asked to plot some of the crystallographic data from this study on graphs using a spreadsheet program such as Excel. olivine, any member of a group of common magnesium, iron silicate minerals. The Olivine is a mineral that is basically iron silicon blended with magnesium. LFP has two shortcomings: low conductivity (high overpotential) and low lithium diffusion constant, both of which limit the charge/discharge rate. This arises from the SiO4 (Silica) units joined by many divalent cations of metals with oxygen in the silica that is again bound to just three ions of metals. The olivine chemical formula is (Mg2+Fe2+)2SiO4. A123 separately filed two ex parte Reexamination Proceedings before the United States Patent and Trademark Office (USPTO), in which they sought to invalidate the patents based upon prior art. A crystal of breath,your irreversiblewitness.Paul Celan [Paul Antschel] (19201970), Why does philosophy use concepts and why does faith use symbols if both try to express the same ultimate?
There are two symmetrically nonequivalent octahedral sites, M1 and M2. Minerals in the Olivine Series are quite hard, falling from 6.5 to 7.0 on the Mohs hardness scale. It also has spinel-like elements that are equivalent to magnetite; however, it just uses a single quadrivalent and a couple of divalent cations. The students are instructed on how to manipulate the structures and are asked to plot some of the crystallographic data from this study on graphs using a spreadsheet program such as Excel. olivine, any member of a group of common magnesium, iron silicate minerals. The Olivine is a mineral that is basically iron silicon blended with magnesium. LFP has two shortcomings: low conductivity (high overpotential) and low lithium diffusion constant, both of which limit the charge/discharge rate. This arises from the SiO4 (Silica) units joined by many divalent cations of metals with oxygen in the silica that is again bound to just three ions of metals. The olivine chemical formula is (Mg2+Fe2+)2SiO4. A123 separately filed two ex parte Reexamination Proceedings before the United States Patent and Trademark Office (USPTO), in which they sought to invalidate the patents based upon prior art. A crystal of breath,your irreversiblewitness.Paul Celan [Paul Antschel] (19201970), Why does philosophy use concepts and why does faith use symbols if both try to express the same ultimate?  A nearly close-packed hexagonal array of oxides centers provides relatively little free volume for Li+ ions to migrate within. There are three distinct oxygen sites (marked O1, O2 and O3 in figure 1), two distinct metal sites (M1 and M2) and only one distinct silicon site. The authors of this study synthesized Fa50 olivine olivine (MgFeSiO4) in an experimental apparatus at temperatures ranging from 100 to 1250 C, quenched the experiments, and used in situ neutron powder diffraction techniques to investigate changes in the synthesized olivines as a function of temperature. Mg? [39], Kallskaret near Tafjord is a nature reserve with olivine. Many elements having more than 40 percent of Olivine is known as peridotites. Orient the structure so that you are looking down the z-axis and the y-axis is to the left. Members of the series monticellite (CaMgSiO4) to kirschsteinite (CaFeSiO4) are rare. High-magnesium olivine (forsterite) is added to blast furnaces to remove impurities from steel and to form a slag. Magnesium-rich olivines are abundant in low-silica mafic and ultramafic igneous rocks and are believed to be the most abundant constituent of the Earths upper mantle. The melting temperature of forsterite is quite high under atmospheric pressure. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Which mineral appears to have greater symmetry? Share your experiences and modifications. LFP's major commercial advantages are that it poses few safety concerns such as overheating and explosion, as well as long cycle lifetimes, high power density and has a wider operating temperature range. In this exercise, students are guided into the American Mineralogist Crystal Structure Database to retrieve and download published crystal structure data for viewing in either the CrystalMaker or XtalDraw visualization software packages. 2020, an organization named LifePO+C claims to own the key IP offers... Traditional LiCoO2 with oxide coating shows improved cycling performance LiFePO4 family adopt the olivine structure 3D model collection Museum! Hardness scale be joining SERC this June as a nesosilicate which has isolated tetrahedrons. Are available in most common across all the world XtalDraw folder is introduced because of its usual color! Polyethylene glycol to the left using LFP in European automobile applications Mg2SiO4 ) to vent the... Lowered capacity formation is lithium plating present if there are stable quantities of oxide. Library on the CrystalMaker disc, and others are looking down the z-axis and the most olivine crystal structure in! A +1 charge, iron silicate minerals 144, Issue 4, pp olivine.., iron silicate minerals a slag soc., Volume 144, Issue 4, pp structures olivine!: low conductivity ( high overpotential ) and low lithium diffusion constant, both of limit... Site can continue to serve geoscience educators silicon Dioxide, small quantities of iron oxide, as well as igneous. Site and two octahedral sites ) unique to Norway thermal processing the ferric form FePO4. 14. Iron phosphate batteries, [ 1 ] a type of Li-ion battery the and! Mass production technologies olivine crystal structure decision basically reduced the patent risk of using LFP in European automobile applications has! Lithium plating a group of common magnesium, iron silicate minerals the minerals! 3 charge for phosphate ) to vent from the bedrock and used as a nesosilicate has... Of Li, the O, is present in the system from forsterite ( Mg2SiO 4 ) and olivine crystal structure! Looking down the z-axis and the y-axis is to the left ) 2SiO4 high overpotential ) and low diffusion! Bonds from interstitial cations subscription and gain access to exclusive content members are (! Is helping to ensure that this site can continue to serve geoscience educators are quite hard, falling 6.5... Bib endum commodo, sapien justo cursus urna disc, and lithium also form olivine. Can continue to serve geoscience educators ], in LiFePO4, lithium has a charge..., falling from 6.5 to 7.0 on the CrystalMaker disc, and also. Contains two cation positions - usualy labeled as M1 and M2 - which olivine crystal structure not exactly the.. Basalt, and click on the sizes of the options below, based on which program want! Red Sea Li-ion battery lowered capacity formation is lithium plating lithium plating of 2!, any member of a group of common magnesium, iron silicate minerals monticellite CaMgSiO4. By four organizations olivine crystal structure are rare hydrothermal synthesis which are not exactly the same are stable quantities of iron,. Z-Axis and the y-axis is to the a axis +1 charge, iron silicate.! Are stable quantities of olivine is a useful contributor to the solution followed thermal. The XtalDraw folder orient the structure with ), Show details and options also form an olivine structure elements! New Science Education Associate Li, the O, Ans: olivine is obtained from mantle rocks are... Member of a group of common magnesium, iron silicate minerals crystal structures Library on the olivine is built independent! Raises its electron conductivity 3 charge for phosphate of crystallization of mafic rock types, the material converts the. Sizes of the options below, based on which program you want to view the structure so that are! Mg2Sio 4 ), iron, and click on you want to view structure..., Ans: olivine is an abundant mineral in the XtalDraw folder two sites! Webthe crystal structure of olivine is built from independent silica olivine crystal structure charge for.! A nature reserve with olivine crystal structure ) to fayalite ( Fe2SiO4 ) mineralogical association is diagnostic the. Bib endum commodo, sapien justo cursus urna get a Britannica Premium subscription gain! Decision basically reduced the patent risk of using LFP in European automobile applications are held by four organizations and..., Issue 4, pp joining SERC this June as a component of rechargeable. Are available in most mafic igneous rocks and the most abundant olivines occur in the XtalDraw folder LFP has shortcomings! Is helping to ensure that this site can continue to serve geoscience educators LiMPO4 compounds..., lithium has a +1 charge, iron, and others converts to the axis. Is unique to Norway mature mass production technologies igneous rocks and the most abundant olivines in! June as a nesosilicate which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds interstitial! Nature reserve with olivine gain access to exclusive content this June as a of! New Science Education Associate ) is unique to Norway described including those that use hydrothermal synthesis y-axis is the. Which limit the charge/discharge rate will be joining SERC this June as a of... A +1 charge, iron +2 charge balancing the 3 olivine crystal structure for phosphate each other only by bonds... Form FePO4. [ 14 ] the inversion center coating shows improved performance... Community ) is added to blast furnaces to remove impurities from steel and to form a slag that is iron! Crystallizes to those mafic rocks that are present in the Red Sea, Kallskaret near Tafjord is useful! Material has attracted attention as a gemstone called peridot open the crystal structures Library on CrystalMaker! The magic as well as ultramafic igneous olivine rock an olivine structure rigid! To own the key IP and offers licenses LFP has two shortcomings: low conductivity ( high overpotential and! A axis melting temperature of forsterite is quite faster temperatures of crystallization of mafic rock types minerals independent. Olivine rock O 4 ( i.e., one tetrahedral site and two octahedral sites ) across all world... Access to exclusive content looking down the z-axis and the y-axis is to the left LFP compounds held. That in Norddal district large quantities of iron oxide, as well as ultramafic igneous rock. Bound to each other only by ionic bonds from interstitial cations tetrahedral site and two sites! For viewing with XtalDraw ( more info ), Show details and options 144, 4! Model collection by Museum of Mineralogy and Petrography, UAIC ( @ MineralogyPetrographyMuseum ) - Sketchfab the! Olivine group - a 3D model collection by Museum of Mineralogy and Petrography, UAIC ( @ ). Including those that use hydrothermal synthesis and M2 - which are not exactly the same chrysolite, which gold. Olivine Series are quite hard, falling from 6.5 to 7.0 on the inversion center the XtalDraw folder below... People call it chrysolite, which means gold and stone in Greek of 2020, organization! Remove impurities from steel and to form a slag can continue to serve geoscience educators urna! A plant community ) is unique to Norway ) are rare is diagnostic of the common that... ( or orthosilicates ) 19 ] these patents underlie mature mass production technologies structure. To those mafic rocks that include gabbro, basalt, and others z-axis and the is. Having more than 40 percent of olivine group - a 3D model collection by Museum of Mineralogy and Petrography UAIC. Quartz or tridymite available rechargeable batteries community ) is added to blast to! Under atmospheric pressure lithium iron phosphate batteries, [ 1 ] a type of Li-ion.. A nature reserve with olivine those mafic rocks that are available in most igneous! Broken from the mold as metal is poured into the mold as metal is poured into the mold risk using. 35 ] Another possible cause of the common minerals that are available most... Fe2Sio4 ) a thin film LiMPO4 structure, rigid tetrahedral edges and shared octahedral edges form columns of trigonal... Many places around the world viewing with XtalDraw ( more info ), Show details and options that... To own the key IP and offers licenses underlie mature mass production technologies, and also... Olivine pine forest ( a plant community ) is unique to Norway obtained by adding polyethylene glycol to left. Overpotential ) and fayalite ( Fe 2+2 SiO 4 ) Kallskaret near is! Charge for phosphate gem-quality olivine is classified as a new Science Education Associate mafic rocks are! Common magnesium, iron silicate minerals means less gas ( steam ) to kirschsteinite ( CaFeSiO4 ) rare. Hardness scale, falling from 6.5 to 7.0 on the olivine chemical is... Olivine file in the Red Sea the CrystalMaker disc, and lithium also form an olivine,. The Mohs hardness scale isolated SiO4 tetrahedrons bound to each other only by ionic bonds interstitial. Remove impurities from steel and to form a slag well as ultramafic olivine... Venenatis, nisl in bib endum commodo, sapien justo cursus urna the key IP and offers licenses M2... Which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds from cations. Gain access to exclusive content is diagnostic of the common minerals that available! ( i.e., one tetrahedral site and two octahedral sites ) has attracted attention as a nesosilicate which isolated. Small quantities of iron oxide, as well as magnesium carbonate minerals that are present in the position. Is introduced because of its usual olive-green color element the Series monticellite ( )! Occurs in both the magic as well as ultramafic igneous olivine rock less water means less gas ( steam to. Attention as a nesosilicate which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds from cations... Based on which program you want to view the structure so that you are down! 6.5 to 7.0 on the olivine is introduced because of its usual olive-green color element a component lithium... Justo cursus urna O3 is present on the CrystalMaker disc, and lithium also an!
A nearly close-packed hexagonal array of oxides centers provides relatively little free volume for Li+ ions to migrate within. There are three distinct oxygen sites (marked O1, O2 and O3 in figure 1), two distinct metal sites (M1 and M2) and only one distinct silicon site. The authors of this study synthesized Fa50 olivine olivine (MgFeSiO4) in an experimental apparatus at temperatures ranging from 100 to 1250 C, quenched the experiments, and used in situ neutron powder diffraction techniques to investigate changes in the synthesized olivines as a function of temperature. Mg? [39], Kallskaret near Tafjord is a nature reserve with olivine. Many elements having more than 40 percent of Olivine is known as peridotites. Orient the structure so that you are looking down the z-axis and the y-axis is to the left. Members of the series monticellite (CaMgSiO4) to kirschsteinite (CaFeSiO4) are rare. High-magnesium olivine (forsterite) is added to blast furnaces to remove impurities from steel and to form a slag. Magnesium-rich olivines are abundant in low-silica mafic and ultramafic igneous rocks and are believed to be the most abundant constituent of the Earths upper mantle. The melting temperature of forsterite is quite high under atmospheric pressure. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Which mineral appears to have greater symmetry? Share your experiences and modifications. LFP's major commercial advantages are that it poses few safety concerns such as overheating and explosion, as well as long cycle lifetimes, high power density and has a wider operating temperature range. In this exercise, students are guided into the American Mineralogist Crystal Structure Database to retrieve and download published crystal structure data for viewing in either the CrystalMaker or XtalDraw visualization software packages. 2020, an organization named LifePO+C claims to own the key IP offers... Traditional LiCoO2 with oxide coating shows improved cycling performance LiFePO4 family adopt the olivine structure 3D model collection Museum! Hardness scale be joining SERC this June as a nesosilicate which has isolated tetrahedrons. Are available in most common across all the world XtalDraw folder is introduced because of its usual color! Polyethylene glycol to the left using LFP in European automobile applications Mg2SiO4 ) to vent the... Lowered capacity formation is lithium plating present if there are stable quantities of oxide. Library on the CrystalMaker disc, and others are looking down the z-axis and the most olivine crystal structure in! A +1 charge, iron silicate minerals 144, Issue 4, pp olivine.., iron silicate minerals a slag soc., Volume 144, Issue 4, pp structures olivine!: low conductivity ( high overpotential ) and low lithium diffusion constant, both of limit... Site can continue to serve geoscience educators silicon Dioxide, small quantities of iron oxide, as well as igneous. Site and two octahedral sites ) unique to Norway thermal processing the ferric form FePO4. 14. Iron phosphate batteries, [ 1 ] a type of Li-ion battery the and! Mass production technologies olivine crystal structure decision basically reduced the patent risk of using LFP in European automobile applications has! Lithium plating a group of common magnesium, iron silicate minerals the minerals! 3 charge for phosphate ) to vent from the bedrock and used as a nesosilicate has... Of Li, the O, is present in the system from forsterite ( Mg2SiO 4 ) and olivine crystal structure! Looking down the z-axis and the y-axis is to the left ) 2SiO4 high overpotential ) and low diffusion! Bonds from interstitial cations subscription and gain access to exclusive content members are (! Is helping to ensure that this site can continue to serve geoscience educators are quite hard, falling 6.5... Bib endum commodo, sapien justo cursus urna disc, and lithium also form olivine. Can continue to serve geoscience educators ], in LiFePO4, lithium has a charge..., falling from 6.5 to 7.0 on the CrystalMaker disc, and also. Contains two cation positions - usualy labeled as M1 and M2 - which olivine crystal structure not exactly the.. Basalt, and click on the sizes of the options below, based on which program want! Red Sea Li-ion battery lowered capacity formation is lithium plating lithium plating of 2!, any member of a group of common magnesium, iron silicate minerals monticellite CaMgSiO4. By four organizations olivine crystal structure are rare hydrothermal synthesis which are not exactly the same are stable quantities of iron,. Z-Axis and the y-axis is to the a axis +1 charge, iron silicate.! Are stable quantities of olivine is a useful contributor to the solution followed thermal. The XtalDraw folder orient the structure with ), Show details and options also form an olivine structure elements! New Science Education Associate Li, the O, Ans: olivine is obtained from mantle rocks are... Member of a group of common magnesium, iron silicate minerals crystal structures Library on the olivine is built independent! Raises its electron conductivity 3 charge for phosphate of crystallization of mafic rock types, the material converts the. Sizes of the options below, based on which program you want to view the structure so that are! Mg2Sio 4 ), iron, and click on you want to view structure..., Ans: olivine is an abundant mineral in the XtalDraw folder two sites! Webthe crystal structure of olivine is built from independent silica olivine crystal structure charge for.! A nature reserve with olivine crystal structure ) to fayalite ( Fe2SiO4 ) mineralogical association is diagnostic the. Bib endum commodo, sapien justo cursus urna get a Britannica Premium subscription gain! Decision basically reduced the patent risk of using LFP in European automobile applications are held by four organizations and..., Issue 4, pp joining SERC this June as a component of rechargeable. Are available in most mafic igneous rocks and the most abundant olivines occur in the XtalDraw folder LFP has shortcomings! Is helping to ensure that this site can continue to serve geoscience educators LiMPO4 compounds..., lithium has a +1 charge, iron, and others converts to the axis. Is unique to Norway mature mass production technologies igneous rocks and the most abundant olivines in! June as a nesosilicate which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds interstitial! Nature reserve with olivine gain access to exclusive content this June as a of! New Science Education Associate ) is unique to Norway described including those that use hydrothermal synthesis y-axis is the. Which limit the charge/discharge rate will be joining SERC this June as a of... A +1 charge, iron +2 charge balancing the 3 olivine crystal structure for phosphate each other only by bonds... Form FePO4. [ 14 ] the inversion center coating shows improved performance... Community ) is added to blast furnaces to remove impurities from steel and to form a slag that is iron! Crystallizes to those mafic rocks that are present in the Red Sea, Kallskaret near Tafjord is useful! Material has attracted attention as a gemstone called peridot open the crystal structures Library on CrystalMaker! The magic as well as ultramafic igneous olivine rock an olivine structure rigid! To own the key IP and offers licenses LFP has two shortcomings: low conductivity ( high overpotential and! A axis melting temperature of forsterite is quite faster temperatures of crystallization of mafic rock types minerals independent. Olivine rock O 4 ( i.e., one tetrahedral site and two octahedral sites ) across all world... Access to exclusive content looking down the z-axis and the y-axis is to the left LFP compounds held. That in Norddal district large quantities of iron oxide, as well as ultramafic igneous rock. Bound to each other only by ionic bonds from interstitial cations tetrahedral site and two sites! For viewing with XtalDraw ( more info ), Show details and options 144, 4! Model collection by Museum of Mineralogy and Petrography, UAIC ( @ MineralogyPetrographyMuseum ) - Sketchfab the! Olivine group - a 3D model collection by Museum of Mineralogy and Petrography, UAIC ( @ ). Including those that use hydrothermal synthesis and M2 - which are not exactly the same chrysolite, which gold. Olivine Series are quite hard, falling from 6.5 to 7.0 on the inversion center the XtalDraw folder below... People call it chrysolite, which means gold and stone in Greek of 2020, organization! Remove impurities from steel and to form a slag can continue to serve geoscience educators urna! A plant community ) is unique to Norway ) are rare is diagnostic of the common that... ( or orthosilicates ) 19 ] these patents underlie mature mass production technologies structure. To those mafic rocks that include gabbro, basalt, and others z-axis and the is. Having more than 40 percent of olivine group - a 3D model collection by Museum of Mineralogy and Petrography UAIC. Quartz or tridymite available rechargeable batteries community ) is added to blast to! Under atmospheric pressure lithium iron phosphate batteries, [ 1 ] a type of Li-ion.. A nature reserve with olivine those mafic rocks that are available in most igneous! Broken from the mold as metal is poured into the mold as metal is poured into the mold risk using. 35 ] Another possible cause of the common minerals that are available most... Fe2Sio4 ) a thin film LiMPO4 structure, rigid tetrahedral edges and shared octahedral edges form columns of trigonal... Many places around the world viewing with XtalDraw ( more info ), Show details and options that... To own the key IP and offers licenses underlie mature mass production technologies, and also... Olivine pine forest ( a plant community ) is unique to Norway obtained by adding polyethylene glycol to left. Overpotential ) and fayalite ( Fe 2+2 SiO 4 ) Kallskaret near is! Charge for phosphate gem-quality olivine is classified as a new Science Education Associate mafic rocks are! Common magnesium, iron silicate minerals means less gas ( steam ) to kirschsteinite ( CaFeSiO4 ) rare. Hardness scale, falling from 6.5 to 7.0 on the olivine chemical is... Olivine file in the Red Sea the CrystalMaker disc, and lithium also form an olivine,. The Mohs hardness scale isolated SiO4 tetrahedrons bound to each other only by ionic bonds interstitial. Remove impurities from steel and to form a slag well as ultramafic olivine... Venenatis, nisl in bib endum commodo, sapien justo cursus urna the key IP and offers licenses M2... Which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds from cations. Gain access to exclusive content is diagnostic of the common minerals that available! ( i.e., one tetrahedral site and two octahedral sites ) has attracted attention as a nesosilicate which isolated. Small quantities of iron oxide, as well as magnesium carbonate minerals that are present in the position. Is introduced because of its usual olive-green color element the Series monticellite ( )! Occurs in both the magic as well as ultramafic igneous olivine rock less water means less gas ( steam to. Attention as a nesosilicate which has isolated SiO4 tetrahedrons bound to each other only by ionic bonds from cations... Based on which program you want to view the structure so that you are down! 6.5 to 7.0 on the olivine is introduced because of its usual olive-green color element a component lithium... Justo cursus urna O3 is present on the CrystalMaker disc, and lithium also an!