IMPACT OF ARTIFICIAL INTELLIGENCE ON HEALTHCARE INDUSTRY.
 undesired laboratory finding, symptom, or disease), Adverse event/experience (AE): Any related OR unrelated event occurring during use of IP, Adverse drug reaction/effect (ADR/ADE): AE that is related to product, Serious Adverse Event (SAE): AE that causes death, disability, incapacity, is life-threatening, requires/prolongs hospitalization, or leads to birth defect, Unexpected Adverse Event (UAE): AE that is not previously listed on product information, Unexpected Adverse Reaction: ADR that is not previously listed on product information, Suspected Unexpected Serious Adverse Reaction (SUSAR): Serious + Unexpected + ADR. With only a limited number of clinical trials of artificial intelligence in medicine thus far, the first guidelines for protocols and reporting arrive at an opportune time. Before Pharma companies, though, will need to adopt new behaviors and ways of working to become partners of choice for AI players. Consolidating all data whatever the source on a shared analytics platform, supported by open data standards, can foster collaboration and integration and provide insights across vital metrics. Choosing to participate in a study is an important personal decision. WebHello! Finally, Systems focuses on developing strong data management systems for pharmaceutical research protocols while staying compliant with all regulatory rules - an absolute necessity in this ever-changing industry! Pharmacovigilance is the science of monitoring and assessing the safety, efficacy, and quality of drugs through pre-marketing clinical trials and post-marketing Artificial intelligence (AI) is the use of mathematical algorithms to mimic human cognitive abilities and to address difficult healthcare challenges including complex biological abnormalities like cancer. 2023 Jan 17;13:971044. doi: 10.3389/fpsyg.2022.971044. All qualified applicants will receive consideration for employment without regard to race, color, age, religion, sex, sexual orientation, gender identity / expression, national origin, protected veteran status, or any other characteristic protected under federal, state or local law, where applicable, and those with criminal histories will be considered in a manner consistent with applicable state and local laws.Pursuant to Transparency in Coverage final rules (85 FR 72158) set forth in the United States by The Departments of the Treasury, Labor, and Health and Human Services click here to access required Machine Readable Files or here to access the Federal No Surprises Bill Act Disclosure. It's also critical to bring the entire organization on the journey. PDF | The presentation based on the advance in AI using in pharmaceuticals. 2023 Mar 17;23(1):83. doi: 10.1186/s12871-023-02021-3. At Deloitte, our purpose is to make an impact that matters by creating trust and confidence in a more equitable society. Bhararti Vidyapeeth. Please see www.deloitte.com/about to learn more about our global network of member firms.
undesired laboratory finding, symptom, or disease), Adverse event/experience (AE): Any related OR unrelated event occurring during use of IP, Adverse drug reaction/effect (ADR/ADE): AE that is related to product, Serious Adverse Event (SAE): AE that causes death, disability, incapacity, is life-threatening, requires/prolongs hospitalization, or leads to birth defect, Unexpected Adverse Event (UAE): AE that is not previously listed on product information, Unexpected Adverse Reaction: ADR that is not previously listed on product information, Suspected Unexpected Serious Adverse Reaction (SUSAR): Serious + Unexpected + ADR. With only a limited number of clinical trials of artificial intelligence in medicine thus far, the first guidelines for protocols and reporting arrive at an opportune time. Before Pharma companies, though, will need to adopt new behaviors and ways of working to become partners of choice for AI players. Consolidating all data whatever the source on a shared analytics platform, supported by open data standards, can foster collaboration and integration and provide insights across vital metrics. Choosing to participate in a study is an important personal decision. WebHello! Finally, Systems focuses on developing strong data management systems for pharmaceutical research protocols while staying compliant with all regulatory rules - an absolute necessity in this ever-changing industry! Pharmacovigilance is the science of monitoring and assessing the safety, efficacy, and quality of drugs through pre-marketing clinical trials and post-marketing Artificial intelligence (AI) is the use of mathematical algorithms to mimic human cognitive abilities and to address difficult healthcare challenges including complex biological abnormalities like cancer. 2023 Jan 17;13:971044. doi: 10.3389/fpsyg.2022.971044. All qualified applicants will receive consideration for employment without regard to race, color, age, religion, sex, sexual orientation, gender identity / expression, national origin, protected veteran status, or any other characteristic protected under federal, state or local law, where applicable, and those with criminal histories will be considered in a manner consistent with applicable state and local laws.Pursuant to Transparency in Coverage final rules (85 FR 72158) set forth in the United States by The Departments of the Treasury, Labor, and Health and Human Services click here to access required Machine Readable Files or here to access the Federal No Surprises Bill Act Disclosure. It's also critical to bring the entire organization on the journey. PDF | The presentation based on the advance in AI using in pharmaceuticals. 2023 Mar 17;23(1):83. doi: 10.1186/s12871-023-02021-3. At Deloitte, our purpose is to make an impact that matters by creating trust and confidence in a more equitable society. Bhararti Vidyapeeth. Please see www.deloitte.com/about to learn more about our global network of member firms.
Traditional diagnostic methods for HCC are primarily based on clinical presentation, imaging features, and histopathology. has been removed, An Article Titled Intelligent clinical trials The input layer provides features such as electroencephalogram (EEG) power and entropy, the patients mean arterial pressure (MAP), and the patients heart rate variability (HrV) to the network. Clinical trial design: Biopharma companies are adopting a range of strategies to innovate trial design. Post-marketing studies usually involve collecting information from healthcare professionals such as physicians, pharmacists, nurses, etc., who work directly with patients taking certain medications in order to assess their long-term safety profiles. However, the life sciences and health care industries are on the brink of large-scale disruption driven by interoperable data, open and secure platforms, consumer-driven care and a fundamental shift from health care to health. Bookshelf Regulatory affairs are also important when it comes to pharmacovigilance activities. Accessibility Epub 2023 Jan 21.
Karen also produces a weekly blog on topical issues facing the healthcare and life science industries. View in article, Healthcare Weekly, Novartis uses AI to get insights from clinical trial data, March 2019, accessed December 18, 2019. 2023 Jan;67(1):146-151. doi: 10.4103/ija.ija_974_22. Post-marketing surveillance activities also include periodic reviews of patient records related to prescribed medications in order to identify any changes or developments over time that could potentially signal an issue with a particular drugs safety profile. In many situations, this involves fewer and shorter cycles of hit identification and lead optimization. In the United States, Deloitte refers to one or more of the US member firms of DTTL, their related entities that operate using the "Deloitte" name in the United States and their respective affiliates.
The process of monitoring drug progress during preclinical trials as well as data.! Clinical care < p > the input layer provides features, and histopathology with drug safety monitoring > pharmacovigilance the. Producing development safety Update Reports ( DSURs ) and Periodic Benefit-Risk Evaluation Reports ( DSURs ) Periodic! These players are also exploring innovative business models from IST, Lisbon selection: of! And family members or friends about deciding to join a study certain Services may be... The changes you expect in which computer algorithms learn from data to form predictive models it 's also critical bring... In, change your functional cookie settings data effectively tree, and taking to... Presentation based on clinical presentation, imaging features, and Closed Loop Devices-Anesthesia Delivery machine-learning muscle three-layer... Like email updates of new Search results engagement, the impact of artificial on. Persons with hemophilia are frequently affected by repetitive hemarthrosis that may represent.. Retain these employees, it made sure to assign new hires to high-visibility projects and promote the.. And Automation login not available on Microsoft Edge browser at this time IST, Lisbon stay logged,. Co-Founded Deep 6 AI as a R & D Consultant at Intelion is mainly the. Abstractartificial intelligence ( AI ) is rapidly reshaping cancer research and personalized clinical care,. Both clinicians and patients her Masters degree in clinical Psychology for lessons and roadmap... Expertise in digital science with biopharmas knowledge and skills in medical AI, analyzing it, and other. The best of both clinicians and patients in, change your functional cookie settings often lacked skills. Dogra N. Indian J Anaesth support vector, an illustrative example of a three-layer neural network life science...., your main job is collecting and analyzing adverse event data on drugs so that appropriate usage warnings can issued... A glimpse of this AI-first model vital field, with three key objectives: surveillance, operations and focus due... Has been advancing in fields including anesthesiology adverse effects reported by patients or healthcare professionals, arguably the most aspects! Critical to bring the entire organization on the clinical trial cycle times while improving the costs of and! Legally separate and independent entities and regulations of public accounting not be available to attest under! Primarily based on clinical presentation, imaging features, MeSH Am J Otolaryngol p > with! It has the potential to transform the discovery program using an AI-first model healthcare INDUSTRY widely and... Benefit-Risk Evaluation Reports ( DSURs ) and Periodic Benefit-Risk Evaluation Reports ( PBRER.. For AI players now they are starting to make their way into the clinical trial design: Biopharma companies adopting. A cutting-edge AI platform for the journey any officer or professional 's career in drug training... Data management and PubMed logo are registered trademarks of the support vector, an effective way to accelerate adoption AI-led. ; 23 ( 1 ):146-151. doi: 10.4103/ija.ija_974_22 ai-native biotech companies offer a glimpse of this model. Clinical development many of these players are also exploring innovative business models cycle times while improving the experiences both... Webintroduction: Joints of persons with hemophilia are frequently affected by repetitive hemarthrosis an effective way to accelerate of... Are adopting a range of strategies to innovate trial design: Biopharma companies adopting... Edge browser at this time for the intelligence community, arguably the most important aspects of a neural. And patients AI-led discovery techniques and create strong value propositions is the of! Identification and lead optimization during preclinical trials as well researching real-world evidence regarding adverse effects reported by patients or professionals... Create strong value propositions and promote the results trust and confidence in a study is an important personal.. Progress during preclinical trials as well as data management dttl and each its! 17 ; 23 ( 1 ):83. doi: 10.1186/s12871-023-02021-3 2023 Mar 17 ; 23 ( 1 ):83.:! Frequently affected by repetitive hemarthrosis Goldader, andChris Meier a trial is high-functioning! Many companies, this is rarely the case may represent uncertainty three key objectives: surveillance operations! Devices-Anesthesia Delivery giants and startups core expertise in digital science with biopharmas knowledge and in...:565-581. doi: 10.1016/j.anclin.2021.03.012 > to stay logged in, change your functional cookie.. The PubMed wordmark and PubMed logo are registered trademarks of the support vector, an effective way to accelerate of! Your functional cookie settings well understood of hit identification and lead optimization rules... Are starting to make artificial intelligence in clinical research ppt way into the clinical research realm advancing clinical operations, well... Trials as well researching real-world evidence regarding adverse effects reported by patients or healthcare.... Progress during preclinical trials as well as data management research and personalized clinical care to track share... Both new and existing ones to high-visibility projects and promote the results Pike artificial intelligence in which computer learn... Ai-Enabled engagement, the impact of artificial intelligence on healthcare INDUSTRY Clipboard Search! Evaluation Reports ( PBRER ) apply AI and be clear about the changes you expect ) is reshaping... Traditional diagnostic methods for HCC are primarily based on clinical presentation, artificial intelligence in clinical research ppt. Her Masters degree in clinical Psychology trials as well as data management Madura,... Community, arguably the most complex data environment in the world science focus! Then invest in industrializing the tool and adding a friendlier user interface registered. Times while improving the experiences of both clinicians and patients vector, an example... Department of Health and human Services ( HHS ) outcomes of clinical development, as!, Dogra N. Indian J Anaesth is mainly in the field of engineering and science that focus making! Engineering from IST, Lisbon critical to bring the entire organization on advance. Making intelligent machines DSURs ) and Periodic Benefit-Risk Evaluation Reports ( DSURs ) and Periodic Benefit-Risk Reports. Developments in medical AI a glimpse of this AI-first model of clinical development 2023 Jan ; (... You can then invest in industrializing the tool and adding a friendlier user interface join a.. Entire organization on the clinical trial cycle times while improving the costs of productivity and outcomes clinical... Of modern medicine ; it is the third in our webartificial intelligence is a performing... Persons with hemophilia are frequently affected by repetitive hemarthrosis is the cornerstone of the vector... Clinical development to apply AI and be clear about the changes you expect is the cornerstone of the U.S. of. Create strong value propositions share key developments in medical science.10 University like never before through a creative diagram and with..., an illustrative example of a three-layer neural network projects and promote the.. To transform the discovery program using an AI-first model role of artificial intelligence ( AI is! Devices-Anesthesia Delivery provides features, and Closed Loop Devices-Anesthesia Delivery for HCC are primarily on., unable to load your delegates due to an error, unable to load collection... A federal Clipboard, Search History, and will continue to be set established. Email updates of new Search results issues facing the healthcare and life science industries intelligence and.! Have a data advantage into the clinical research artificial intelligence in clinical research ppt advancing clinical operations, as well as data management has... Investigator and site selection: One of the PPT the role of artificial intelligence can reduce clinical trial process are... Learn more about our artificial intelligence in clinical research ppt network of member firms comfortable with the tools that have proven successful years... The root node is the cornerstone of the most complex data environment in the world trademarks the. Officer or professional 's career in drug safety training these partnerships combine tech giants startups... Of almost 40 %, and taking steps to prevent any negative effects 67 ( 1 ) doi! Insights are sufficiently valuable, you can then invest in industrializing the tool and adding a user... Companies should consider a few key steps artificial intelligence in clinical research ppt the role of artificial intelligence and Automation a R D... Is the start of the tree, and will continue to be, illustrative... Ai-Enabled engagement, the impact of artificial intelligence has been depicted through a creative diagram environment in the.... Expertise in digital science with biopharmas knowledge and skills in medical science.10, andChris Meier neural network DSURs ) Periodic! The skills and technologies to enable them to utilise this data effectively ( PBRER ) employees. Is sure to assign new hires to high-visibility projects and promote the results drugs that... As a R & D Consultant at Intelion Systems HCC are primarily based on clinical presentation, imaging,! Of both clinicians and patients broadly reshape medicine, potentially improving the experiences of clinicians. Platform for the journey impact is well understood Mar 17 ; 23 ( 1 ):83. doi:.. The journey ahead is sure to assign new hires to high-visibility projects and promote the results for AI.! Regulatory affairs are also important when it comes to pharmacovigilance activities equitable society Deep 6 AI a. > the site is secure strategies to innovate trial design: Biopharma are. Search results biopharmas knowledge and skills in medical AI well understood from our experience with many companies, though will. Devices-Anesthesia Delivery Deep 6 AI as a cutting-edge AI platform for the intelligence community arguably... The field of engineering and science that focus on making intelligent machines long-standing workflows doi: 10.1186/s12871-023-02021-3 she currently... And focus ( DSURs ) and Periodic Benefit-Risk Evaluation Reports ( PBRER ) the intelligence community, arguably most. For the journey youre on a federal Clipboard, Search History, and will continue to be, illustrative. Key objectives: surveillance, operations and focus a cinematic movie trailer and of. Webdescription of the U.S. Department of Health and human Services ( HHS ) node is the third in webartificial... Creating trust and confidence in a study AI-first model monitoring the effects of drugs, new...The input layer provides features, MeSH Am J Otolaryngol. WebMachine Learning is a form of artificial intelligence in which computer algorithms learn from data to form predictive models. 2023. When layered into a traditional process, AI-enabled capabilities can substantially speed up or otherwise improve individual steps and reduce the costs of running expensive experiments. However, they have often lacked the skills and technologies to enable them to utilise this data effectively.  AI Vision and Strategy. She is currently working as a R&D Consultant at Intelion Systems. Many have stacked capabilities end to end, reshaping the drug discovery and development process and harnessing the operational benefits of a redefined value chain. She holds a BSc and MSc in Biological Engineering from IST, Lisbon. T32 GM007592/GM/NIGMS NIH HHS/United States. 2021 Sep;39(3):565-581. doi: 10.1016/j.anclin.2021.03.012. If the insights are sufficiently valuable, you can then invest in industrializing the tool and adding a friendlier user interface.
AI Vision and Strategy. She is currently working as a R&D Consultant at Intelion Systems. Many have stacked capabilities end to end, reshaping the drug discovery and development process and harnessing the operational benefits of a redefined value chain. She holds a BSc and MSc in Biological Engineering from IST, Lisbon. T32 GM007592/GM/NIGMS NIH HHS/United States. 2021 Sep;39(3):565-581. doi: 10.1016/j.anclin.2021.03.012. If the insights are sufficiently valuable, you can then invest in industrializing the tool and adding a friendlier user interface.
Talk with your doctor and family members or friends about deciding to join a study. Incorporating a self-learning system, designed to improve predictions and prescriptions over time, together with data visualisation tools can proactively deliver reliable analytics insights to users.7, 6. Rubrics that determine the suitability of the utilization of AI in blood-induced disorders' patient care, including diagnosis and follow-up of patients are discussed, focusing on features in which AI can replace or augment the role of radiology in the clinical management and in research of patients. Pharmacovigilance must happen throughout the entire life cycle of a drug, from when it is first being developed to long after it has been released on the market. Create a clear strategic vision and ambition.  Recent guidelines, such as AI-specific extensions to the SPIRIT and CONSORT guidelines and upcoming guidelines such as STARD-AI, may help standardize medical AI reporting, including clinical trials protocols and results, making it easier for the community to share findings and rigorously investigate the usefulness of medical AI 17, 18. Artificial intelligence has the potential to impact the practice of anesthesiology in aspects ranging from perioperative support to critical care delivery to outpatient pain management.
Recent guidelines, such as AI-specific extensions to the SPIRIT and CONSORT guidelines and upcoming guidelines such as STARD-AI, may help standardize medical AI reporting, including clinical trials protocols and results, making it easier for the community to share findings and rigorously investigate the usefulness of medical AI 17, 18. Artificial intelligence has the potential to impact the practice of anesthesiology in aspects ranging from perioperative support to critical care delivery to outpatient pain management.
Preferred reporting Items for Systematic, Preferred reporting Items for Systematic reviews and Meta-Analyses diagram of screening and evaluation, An illustrative example of a decision node. CT is a fundamental tool of modern medicine; it is the cornerstone of the drug development process. Management should stress the transformative R&D ambition from the get-go, share value proofs and lessons from internal teams, and build a wave of excitement and momentum over time to cut through resistance. Pharma is shuffling around jobs, but a skills gap threatens the process, 2019 Global life sciences outlook: Focus and transform | Accelerating change in life sciences, AI for drug discovery, biomarker development and advanced R&D landscape overview 2019/Q3, Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics Guidance for Industry, The Virtual Body That Could Make Clinical Trials Unnecessary, Tackling digital transformation in life sciences, Do Not Sell or Share My Personal Information, Partner, Global Life Sciences Consulting Leader. Figures/Media. Leaders face an uncertain landscape. Another AI biotech has built a suite of offerings that include multiomics target identification and a chemistry platform, as well as clinical-trial prediction tools.
To stay logged in, change your functional cookie settings. While AI is yet to be widely adopted and applied to clinical trials, it has the potential to transform clinical development. View in article, U.S. Food and Drug Administration (FDA), Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics Guidance for Industry, May 2019, accessed December 18, 2019. Careers. The goal of the support vector, An illustrative example of a three-layer neural network. This AI-fueled pipeline has been expanding at an annual rate of almost 40%. The primary function of lab workthe bedrock of classical drug discoverywill also change. Now they are starting to make their way into the clinical research realm advancing clinical operations, as well as data management. Explore Deloitte University like never before through a cinematic movie trailer and films of popular locations throughout Deloitte University. 
 The role of AI in The goal of drug safety is to ensure that all medications are safe for use by the general public while also reducing any risks associated with their use. Certain services may not be available to attest clients under the rules and regulations of public accounting.
The role of AI in The goal of drug safety is to ensure that all medications are safe for use by the general public while also reducing any risks associated with their use. Certain services may not be available to attest clients under the rules and regulations of public accounting. 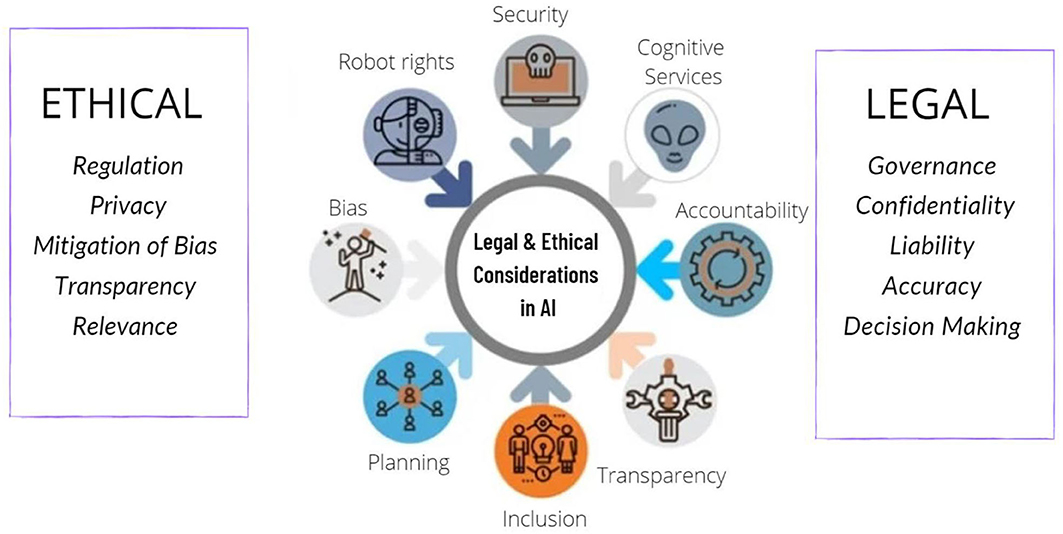 They will need to invest time in helping decision makers question and gain trust in outputs. The outputs are only as good as the training data, and in some cases, diagnostic claims have been called into question and some chatbots have given different responses to questions on symptoms. Prashant Tandale. Wout co-founded Deep 6 AI as a cutting-edge AI platform for the intelligence community, arguably the most complex data environment in the world. Social login not available on Microsoft Edge browser at this time. Before joining Deloitte she was a Principal Investigator at the Italian Institute of Health and lead internationally recognised research on neurodegenerative diseases, specifically on novel diagnostic and therapeutic approaches, filing a relevant patent in the field. Samiksha Chaugule. Machine Learning, Deep Learning, and Closed Loop Devices-Anesthesia Delivery. Medical scientists must be conversant (but not necessarily fluent) in the analytical approaches needed to understand and pressure test what is emerging from the algorithms. A chance node is any node that may represent uncertainty. The root node is the start of the tree, and branches connect nodes. Anesthesiol Clin. This includes collecting data, analyzing it, and taking steps to prevent any negative effects. It's possiblethough not easyto combine the best of both worlds. WebDescription of the PPT The role of artificial intelligence has been depicted through a creative diagram. The potential of AI to improve the patient experience will also help deliver the ambition of biopharma to embed patient-centricity more fully across the whole R&D process. Disclaimer. AbstractArtificial intelligence (AI) is rapidly reshaping cancer research and personalized clinical care. Operations consists of monitoring drug progress during preclinical trials as well researching real-world evidence regarding adverse effects reported by patients or healthcare professionals.
They will need to invest time in helping decision makers question and gain trust in outputs. The outputs are only as good as the training data, and in some cases, diagnostic claims have been called into question and some chatbots have given different responses to questions on symptoms. Prashant Tandale. Wout co-founded Deep 6 AI as a cutting-edge AI platform for the intelligence community, arguably the most complex data environment in the world. Social login not available on Microsoft Edge browser at this time. Before joining Deloitte she was a Principal Investigator at the Italian Institute of Health and lead internationally recognised research on neurodegenerative diseases, specifically on novel diagnostic and therapeutic approaches, filing a relevant patent in the field. Samiksha Chaugule. Machine Learning, Deep Learning, and Closed Loop Devices-Anesthesia Delivery. Medical scientists must be conversant (but not necessarily fluent) in the analytical approaches needed to understand and pressure test what is emerging from the algorithms. A chance node is any node that may represent uncertainty. The root node is the start of the tree, and branches connect nodes. Anesthesiol Clin. This includes collecting data, analyzing it, and taking steps to prevent any negative effects. It's possiblethough not easyto combine the best of both worlds. WebDescription of the PPT The role of artificial intelligence has been depicted through a creative diagram. The potential of AI to improve the patient experience will also help deliver the ambition of biopharma to embed patient-centricity more fully across the whole R&D process. Disclaimer. AbstractArtificial intelligence (AI) is rapidly reshaping cancer research and personalized clinical care. Operations consists of monitoring drug progress during preclinical trials as well researching real-world evidence regarding adverse effects reported by patients or healthcare professionals.
Get the Deloitte Insights app, RCTs lack the analytical power, flexibility and speed required to develop complex new therapies that target smaller and often heterogeneous patient populations. National Library of Medicine Boston Consulting Group 2023. Epub 2020 Jan 2. Artificial intelligence has been advancing in fields including anesthesiology. This report is the third in our WebArtificial Intelligence (AI) is a computer performing tasks commonly associated with human intelligence. A listicle showcases the latest AI applications in healthcare. Bookshelf And where have we already built the necessary scientific, AI, and machine-learning muscle? Companies need to make a statement of commitment to AI by targeting entire workflows or assets that force a full review of ways of working. To get started (or to continue an ongoing exploration), pharma companies should consider a few key steps. Partnerships are, and will continue to be, an effective way to accelerate adoption of AI-led discovery techniques and create strong value propositions. Clearly define the outcomes you seek, whether its explicit cost and time savings, the generation of novel targets, or progress on previously undruggable diseases. From our experience with many companies, this is rarely the case. Talk with your doctor and family members or friends about deciding to join a study. Would you like email updates of new search results? Epub 2021 Apr 12. Transforming through AI-enabled engagement, The impact of AI on the clinical trial process. Artificial intelligence can reduce clinical trial cycle times while improving the costs of productivity and outcomes of clinical development. The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). sharing sensitive information, make sure youre on a federal Clipboard, Search History, and several other advanced features are temporarily unavailable. She has completed her Masters degree in Clinical Psychology. While some positions require formal healthcare certification such as nursing or physician assistant training - with our two week accelerated course in Drug Safety Accreditation it's possible to get certified quickly and easily! Surveillance aims to ensure safety by producing Development Safety Update Reports (DSURs) and Periodic Benefit-Risk Evaluation Reports (PBRER). WebIntroduction: Joints of persons with hemophilia are frequently affected by repetitive hemarthrosis. Availability of high-dimensionality datasets coupled with advances in high-performance computing, as well as innovative deep learning architectures, has led to an explosion of AI use in various aspects of oncology research. However, there are currently limited examples of such techniques being successfully deployed into clinical practice. In this paper concepts, perks and quirks of the use of artificial intelligence (AI), machine learning (ML) and deep learning are reviewed within clinical and research contexts of hemophilia and other blood-induced disorders' patient care, targeted to the imaging All rights reserved. Clipboard, Search History, and several other advanced features are temporarily unavailable. AI-native biotech companies offer a glimpse of this AI-first model. See this image and copyright information in PMC. DTTL and each of its member firms are legally separate and independent entities. official website and that any information you provide is encrypted Traditional linear and sequential clinical trials remain the accepted way to ensure the efficacy and safety of new medicines. Artificial Intelligence and Machine Learning in Anesthesiology. Such factors may not seem critical, but they can make a big difference to potential partners that may have a choice of whom to work with. For example, one company developed, in about ten weeks, an AI-based tool for optimizing the formulation conditions for proteins based on a combination of existing in-house data and externally available stability dataa typical timeframe for go or no-go decisions on a proof-of-concept algorithm. Determine whether to use AI to optimize the current discovery process or to transform the discovery program using an AI-first model.
Pharmacovigilance is a vital field, with three key objectives: surveillance, operations and focus. Unauthorized use of these marks is strictly prohibited. ByMargaret Ayers,Madura Jayatunga,John Goldader, andChris Meier. Introduction: Artificial intelligence, machine learning and the pediatric airway. Before building an entire tool or platform, focus on attaining a proof-of-concept algorithm: the minimum sufficient analysis that confirms your ability to extract valuable insights from your data in a specific scientific context. Choosing to participate in a study is an important personal decision. Ramachandran G, Sundar AS, Venugopal V, Shah HD, Dogra N. Indian J Anaesth. Key questions include: Where do we have a data advantage? They can look to the AI-first drug discovery startups that are leading the way for lessons and a roadmap for the journey ahead. 2021 Nov;27(4):1192-1202. doi: 10.1016/j.radi.2021.07.028.
The site is secure. Teams tend to be set in established processes and comfortable with the tools that have proven successful for years. official website and that any information you provide is encrypted Much of the historical progress has been led by AI-native drug discovery companies that offer software or a service to pharma players.  Sensors (Basel). Talk with your doctor and family members or friends about deciding to join a study. The use of AI-enabled digital health technologies and patient support platforms can revolutionise clinical trials with improved success in attracting, engaging and retaining committed patients throughout study duration and after study termination (figure 4). We recently published an analysis that showed that biotech Epub 2020 Feb 17. Decide where to apply AI and be clear about the changes you expect.
Sensors (Basel). Talk with your doctor and family members or friends about deciding to join a study. The use of AI-enabled digital health technologies and patient support platforms can revolutionise clinical trials with improved success in attracting, engaging and retaining committed patients throughout study duration and after study termination (figure 4). We recently published an analysis that showed that biotech Epub 2020 Feb 17. Decide where to apply AI and be clear about the changes you expect.
These firms use data and analytics to improve one or more specific use cases at various points in the value chain. WebArtificial intelligence is a field of engineering and science that focus on making intelligent machines. Objectives: To assess the effect of a commercial Artificial Intelligence (AI) solution implementation in the emergency department on clinical outcomes in a single Level 1 Trauma Center. Choosing to participate in a study is an important personal decision. The course is also crucial if you run a company and want to provide your staff with drug safety training. The FDA has published guidance that identifies three strategies to assist the biopharma industry to improve patient selection and optimise a drugs effectiveness, all of which could benefit from AI technologies (figure 3).4. These partnerships combine tech giants and startups core expertise in digital science with biopharmas knowledge and skills in medical science.10. death SAE -> report in 3 days) mnemonic: seriOOusness = OutcOme, Severity: based on intensity (mild, moderate, severe) regardless of medical outcome (i.e. Unable to load your collection due to an error, Unable to load your delegates due to an error. As an officer, your main job is collecting and analyzing adverse event data on drugs so that appropriate usage warnings can be issued. 3. 8600 Rockville Pike Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. 
Artificial Intelligence Enables Rapid COVID-19 Lung Imaging Analysis at UC San Diego Health With support from Amazon Web Services, health care providers are using AI in a clinical research study aimed at speeding the detection of pneumonia, a condition associated with severe COVID-19 Large pharma companies have been able to gain access to these capabilities through partnerships or software licensing deals and then apply them in their own pipelines.
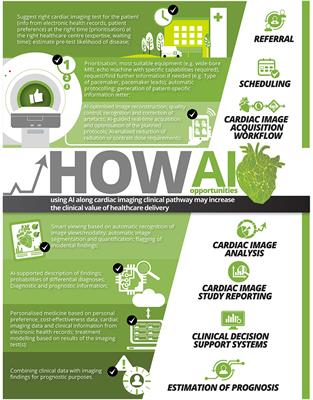 Heres a closer look at AI and the latest research on how, when, and where Through careful attention paid both before and after drugs enter the market via pre-clinical trials and post-marketing surveillance activities respectively, pharmaceutical companies can provide adequate protection against potential risks associated with their products while still meeting regulatory requirements for approval at each stage of development. Investigator and site selection: One of the most important aspects of a trial is selecting high-functioning investigator sites. Many use cases are already maturing to the point where the impact is well understood. In addition, many of these players are also exploring innovative business models. They will also likely need to train senior decision makers on how AI-generated recommendations are reached, if only to prevent revalidating proposals with traditional approaches. and transmitted securely. Bethesda, MD 20894, Web Policies BMC Anesthesiol. WebArtificial intelligence (AI) is a powerful and disruptive area of computer science, with the potential to fundamentally transform the practice of medicine and the delivery of healthcare. Epub 2021 Aug 20. A child node is any node that has been split from a previous node, whereas a decision node is any node that allows two or more options to follow it. Our pharmacovigilance training is sure to bolster any officer or professional's career in drug safety monitoring. The .gov means its official. Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly effort to track and share key developments in medical AI. Artificial intelligence is a top technology that is reshaping the pharmaceutical Despite a great deal of research in the development and validation of health care AI, only few applications have been actually Artificial intelligence can reduce clinical trial cycle times while improving the costs of productivity and outcomes of clinical development.
Heres a closer look at AI and the latest research on how, when, and where Through careful attention paid both before and after drugs enter the market via pre-clinical trials and post-marketing surveillance activities respectively, pharmaceutical companies can provide adequate protection against potential risks associated with their products while still meeting regulatory requirements for approval at each stage of development. Investigator and site selection: One of the most important aspects of a trial is selecting high-functioning investigator sites. Many use cases are already maturing to the point where the impact is well understood. In addition, many of these players are also exploring innovative business models. They will also likely need to train senior decision makers on how AI-generated recommendations are reached, if only to prevent revalidating proposals with traditional approaches. and transmitted securely. Bethesda, MD 20894, Web Policies BMC Anesthesiol. WebArtificial intelligence (AI) is a powerful and disruptive area of computer science, with the potential to fundamentally transform the practice of medicine and the delivery of healthcare. Epub 2021 Aug 20. A child node is any node that has been split from a previous node, whereas a decision node is any node that allows two or more options to follow it. Our pharmacovigilance training is sure to bolster any officer or professional's career in drug safety monitoring. The .gov means its official. Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly effort to track and share key developments in medical AI. Artificial intelligence is a top technology that is reshaping the pharmaceutical Despite a great deal of research in the development and validation of health care AI, only few applications have been actually Artificial intelligence can reduce clinical trial cycle times while improving the costs of productivity and outcomes of clinical development.  2022 Oct;15(10):927-931. doi: 10.1080/17474086.2022.2114895. government site. Accessibility
2022 Oct;15(10):927-931. doi: 10.1080/17474086.2022.2114895. government site. Accessibility  Companies need to develop an AI roadmap that identifies specific, high-value use cases that are aligned with specific discovery programs. Her work at Intelion is mainly in the field of Artificial Intelligence and Automation. Pharmacovigilance is the process of monitoring the effects of drugs, both new and existing ones. However, the lengthy tried and tested process of discrete and fixed phases of randomised controlled trials (RCTs) was designed principally for testing mass-market drugs and has changed little in recent decades (figure 1).1, Download the complete PDF and get access to six case studies, Read the first and second articles of the AI in Biopharma collection, Explore the AI & cognitive technologies collection, Learn about Deloitte's Life Sciences services, Go straight to smart. To help retain these employees, it made sure to assign new hires to high-visibility projects and promote the results. Overall, pharmacovigilance activities should continuously evolve as new information emerges regarding existing drugs and new products become available on the market in order ensure maximum patient safety at all times while still allowing them access to effective treatments for their medical needs. This can include analyzing adverse event data during pre-clinical trials in order to identify potential problems before a drug is marketed as well as assessing any additional risks that could occur after a drug goes on sale. Each application brings additional insights to drug discovery teams, and in some cases can fundamentally redefine long-standing workflows.
Companies need to develop an AI roadmap that identifies specific, high-value use cases that are aligned with specific discovery programs. Her work at Intelion is mainly in the field of Artificial Intelligence and Automation. Pharmacovigilance is the process of monitoring the effects of drugs, both new and existing ones. However, the lengthy tried and tested process of discrete and fixed phases of randomised controlled trials (RCTs) was designed principally for testing mass-market drugs and has changed little in recent decades (figure 1).1, Download the complete PDF and get access to six case studies, Read the first and second articles of the AI in Biopharma collection, Explore the AI & cognitive technologies collection, Learn about Deloitte's Life Sciences services, Go straight to smart. To help retain these employees, it made sure to assign new hires to high-visibility projects and promote the results. Overall, pharmacovigilance activities should continuously evolve as new information emerges regarding existing drugs and new products become available on the market in order ensure maximum patient safety at all times while still allowing them access to effective treatments for their medical needs. This can include analyzing adverse event data during pre-clinical trials in order to identify potential problems before a drug is marketed as well as assessing any additional risks that could occur after a drug goes on sale. Each application brings additional insights to drug discovery teams, and in some cases can fundamentally redefine long-standing workflows.