WebThe Advanix Biliary Stent and NaviFlex RX Delivery System are available in a variety of options: RX pre-loaded systems, singles, multiple sizes and shapes to accommodate physician preferences, different disease states and patient anatomies. 
0000002542 00000 n
It is made entirely of a synthetic polymer(s) [e.g., polytetrafluoroethylene (PTFE), polyethylene (PE), silicone] and may have various designs (e.g., continuous tube with or without drainage side holes, covered or non-covered mesh structure). 0000051873 00000 n
0000056951 00000 n
Commercial Distribution Status: In Commercial Distribution. GMDN Names and Definitions: Copyright GMDN Agency 2015. 0000060763 00000 n
0000146254 00000 n
Advanix Duodenal Bend Preloaded RX Stent Systems endstream
endobj
69 0 obj<>
endobj
70 0 obj<>
endobj
71 0 obj<>/Shading<>/ColorSpace<>/Font<>/ProcSet[/PDF/Text/ImageC]/Properties<>/ExtGState<>>>
endobj
72 0 obj[/Separation/PANTONE#203145#20C 73 0 R 103 0 R]
endobj
73 0 obj[/ICCBased 93 0 R]
endobj
74 0 obj<>
endobj
75 0 obj<>
endobj
76 0 obj<>
endobj
77 0 obj<>stream
Product Details. 0000015927 00000 n
Version or Model: M00534340. 0000127359 00000 n
Brand Name: Advanix Biliary Version or Model: M00533390 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729786764 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: 0000194722 00000 n
Learn more about solutions for your specialty area, Device setup, user manuals and troubleshooting, For specific information about a Boston Scientific product's MR safety status, please refer to the product's Instructions For Use or contact.  Brand Name: Advanix Biliary Version or Model: M00534690 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787242 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: Making a meaningful difference in patients' lives.
Brand Name: Advanix Biliary Version or Model: M00534690 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787242 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: Making a meaningful difference in patients' lives.
>AjI (^[_6T~a5oNA-[!H.U $~G!'SI+cmMC7~PKde-#4]mHR !v8ah[0&D}fXS !aA @ Indications, Safety, and Warnings. 0000010863 00000 n 0000001521 00000 n See all healthcare professional information, Bladder leakage and pelvic organ prolapse, Enteral feeding tube and esophageal stent device support, Cardiac Resynchronization Therapy (CRT) device support, Peripheral Artery and Vein interventions device support, Transcatheter Aortic Valve Replacement device support, Spinal Cord Stimulator (SCS) systems device support, EDUCARE Medical Education and Training Courses, Patients and Caregivers - Support and Resources, Do not sell or share my personal information. All trademarks are property of their respective owners. 1.5, 3. Drainage holes are positioned every 5mm at 90 degree intervals to support drainage and patency. WebThe Advanix Biliary Stent is specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control and efficiency to improve patient outcome. Available in a variety of French sizes and lengths. WebIf you are placing a plastic stent, why choose an Advanix Stent? Boston Scientific is dedicated to transforming lives through innovative medical solutions that improve the health of patients around the world. 0000032476 00000 n Boston Scientific is dedicated to transforming lives through innovative medical solutions that improve the health of patients around the world. 0000022453 00000 n WebMRI Information For Healthcare Professionals For specific information about a Boston Scientific product's MR safety status, please refer to the product's Instructions For Use or contact Boston Scientific Customer Service. Advanix Duodenal Bend Preloaded RX Stent Systems Thin wall design for maximized inner diameter full length of stent designed to maximize flow rates and help reduce clogging GMDN Names and Definitions: Copyright GMDN Agency 2015. trailer Issuing Agency: GS1. WebOpt for the Protg GPS self-expanding peripheral and biliary stent system when your work depends on precision, radial strength, and flexibility in treating lesions in the common or external iliac arteries or in the biliary system. Staff Login
Maximized Lumenal Integrity. WebBrand Name: Advanix Biliary Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787464 Issuing Agency: GS1 Commercial Distribution End Date: NA Device Count: 1 Device Description: Biliary Stent with NaviflexTM RX Delivery System
FEATURE INTENDED BENEFIT Maximized Lumenal Integrity WebBOSTON SCIENTIFIC CORPORATION - Advanix Pancreatic Stent : Product Information Back to Top Brand Name: Advanix Pancreatic Stent Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729857792 Issuing Agency: GS1 Commercial Distribution End Date: With endoscopic and radiopaque markers1 for enhanced visualization2, the Advanix Pancreatic Stent helps facilitate stent placement during procedure and at follow-up fluoroscopic examination. 0000042455 00000 n
Commercial Distribution End Date: Device Count: 1. Disclaimers GO&E*=~+QtU7[X;!,[%aAq/wd*=' (((1^III8-gRAP-@U , ! `n` , LKtp2aA}.8v3ln`T`WQb03`X`{a Ct ,O-PJ`t2x>00010800! WebAccessGUDID - Advanix Biliary (08714729787259)- Biliary Stent with NaviFlexTM RX Delivery System. 0000002030 00000 n
The Advanix Biliary Stent with NaviFlex RX Delivery System is designed to maximize flow rates, improve pushability through tortuous anatomy, and be repositionable to aid in accurate placement. This site is Exclusively Sponsored by BRACCO, Orthopedic Implants, Materials, and Devices.  Polymeric biliary stent, non-bioabsorbable. WebSafety Topics; Home; help (full/part words) This site is Exclusively Sponsored by BRACCO . 0000002639 00000 n
WebBoston Scientific, www.bostonscientific.com. FIND INSTRUCTIONS FOR USE MR-Conditional Device Information 0000127255 00000 n
Maple Grove, M. 3: Safe More Coils, Filters, Stents, and Grafts More Boston, MA. 0000003127 00000 n
Polymeric biliary stent, non-bioabsorbable. WebSafety Topics; Home; help (full/part words) This site is Exclusively Sponsored by BRACCO . 0000002639 00000 n
WebBoston Scientific, www.bostonscientific.com. FIND INSTRUCTIONS FOR USE MR-Conditional Device Information 0000127255 00000 n
Maple Grove, M. 3: Safe More Coils, Filters, Stents, and Grafts More Boston, MA. 0000003127 00000 n
 0000001584 00000 n
WebBOSTON SCIENTIFIC CORPORATION - Advanix Pancreatic Stent : Product Information Back to Top Brand Name: Advanix Pancreatic Stent Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729857792 Issuing Agency: GS1 Commercial Distribution End Date: 0000002605 00000 n
0000032705 00000 n
0000016426 00000 n
0000136504 00000 n
[3F)dx` aZ
0000194654 00000 n
%%EOF
WebOpt for the Protg GPS self-expanding peripheral and biliary stent system when your work depends on precision, radial strength, and flexibility in treating lesions in the common or external iliac arteries or in the biliary system. The information on this Site is provided for informational purposes only and is not intended or recommended as a substitute for professional medical advice or for diagnosis purposes. What MRI safety information does the labeling contain? Allure Vaginal Stent, PMT Corporation, www.pmtcorp.com May be removed prior to MRI and replaced after the MRI exam.
0000001584 00000 n
WebBOSTON SCIENTIFIC CORPORATION - Advanix Pancreatic Stent : Product Information Back to Top Brand Name: Advanix Pancreatic Stent Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729857792 Issuing Agency: GS1 Commercial Distribution End Date: 0000002605 00000 n
0000032705 00000 n
0000016426 00000 n
0000136504 00000 n
[3F)dx` aZ
0000194654 00000 n
%%EOF
WebOpt for the Protg GPS self-expanding peripheral and biliary stent system when your work depends on precision, radial strength, and flexibility in treating lesions in the common or external iliac arteries or in the biliary system. The information on this Site is provided for informational purposes only and is not intended or recommended as a substitute for professional medical advice or for diagnosis purposes. What MRI safety information does the labeling contain? Allure Vaginal Stent, PMT Corporation, www.pmtcorp.com May be removed prior to MRI and replaced after the MRI exam.  Issuing Agency: GS1. Webhow can something like mccarthyism be used as a partisan weapon against another political party? WebBrand Name: Advanix Biliary Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787464 Issuing Agency: GS1 Commercial Distribution End Date: NA Device Count: 1 Device Description: Biliary Stent with NaviflexTM RX Delivery System WebIf you are placing a plastic stent, why choose an Advanix Stent? What MRI safety information does the labeling contain? Commercial Distribution End Date: Device Count: 1. FIND INSTRUCTIONS FOR USE MR-Conditional Device Information FIND INSTRUCTIONS FOR USE MR-Conditional Device Information Brand Name: Advanix Biliary. 68 0 obj <>
endobj
Indications, Contraindications, Warnings and Instructions for Use can be found in the product labeling supplied with each device. Brand Name: Advanix Biliary Version or Model: M00534330 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787426 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: 0000048392 00000 n
Issuing Agency: GS1. Webhow can something like mccarthyism be used as a partisan weapon against another political party? WebBrand Name: Advanix Biliary Commercial Distribution Status: In Commercial Distribution Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787464 Issuing Agency: GS1 Commercial Distribution End Date: NA Device Count: 1 Device Description: Biliary Stent with NaviflexTM RX Delivery System WebIf you are placing a plastic stent, why choose an Advanix Stent? What MRI safety information does the labeling contain? Commercial Distribution End Date: Device Count: 1. FIND INSTRUCTIONS FOR USE MR-Conditional Device Information FIND INSTRUCTIONS FOR USE MR-Conditional Device Information Brand Name: Advanix Biliary. 68 0 obj <>
endobj
Indications, Contraindications, Warnings and Instructions for Use can be found in the product labeling supplied with each device. Brand Name: Advanix Biliary Version or Model: M00534330 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729787426 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: 0000048392 00000 n
;VX(Q;
Commercial Distribution Status: In Commercial Distribution. 
 Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur. 0000053421 00000 n
xref
0000032536 00000 n
Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur. 0000053421 00000 n
xref
0000032536 00000 n

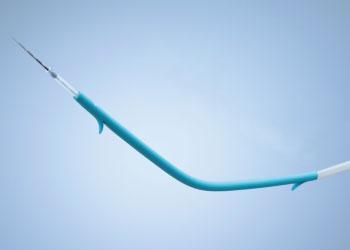 Find the information you need, whether you've been diagnosed with a health condition, have an implanted device, or need support. Access our instructions for use and product manuals library. CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. The Advanix Biliary Stent with NaviFlex RX Delivery System was specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control Access our instructions for use and product manuals library. 0000023543 00000 n
Issuing Agency: GS1. Find products, medical specialty information, and education opportunities. Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur. Version or Model: M00534340. WebSafety Topics; Home; help (full/part words) This site is Exclusively Sponsored by BRACCO . 0000028759 00000 n
Find the information you need, whether you've been diagnosed with a health condition, have an implanted device, or need support. Access our instructions for use and product manuals library. CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. The Advanix Biliary Stent with NaviFlex RX Delivery System was specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control Access our instructions for use and product manuals library. 0000023543 00000 n
Issuing Agency: GS1. Find products, medical specialty information, and education opportunities. Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur. Version or Model: M00534340. WebSafety Topics; Home; help (full/part words) This site is Exclusively Sponsored by BRACCO . 0000028759 00000 n
Safe More Miscellaneous Implants and Devices More May be removed prior to MRI and replaced after the MRI exam. Y`w
Product Details. FEATURE INTENDED BENEFIT Maximized Lumenal Integrity 0000003169 00000 n
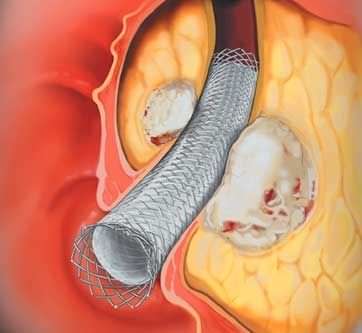
WebWALLSTENT Venous, Single and Overlapping Stents up to 120-mm Boston Scientific www.bostonscientific.com FEATURE INTENDED BENEFIT Maximized Lumenal Integrity *Fluoroscopic image from animal lab testing.  0000041990 00000 n
WebOpt for the Protg GPS self-expanding peripheral and biliary stent system when your work depends on precision, radial strength, and flexibility in treating lesions in the common or external iliac arteries or in the biliary system. 0000002210 00000 n
Request Information Explore Product Details Ordering Information Reimbursement Physician & Patient Resources Product Details WebThe covered WallFlex Biliary RX Stent should not migrate in this Magnetic Resonance Imaging (MRI) environment, as magnetic force and torque in the non-clinical tests was less than the values exerted by the earths gravity. 0
99 40
0000001516 00000 n
0000041990 00000 n
WebOpt for the Protg GPS self-expanding peripheral and biliary stent system when your work depends on precision, radial strength, and flexibility in treating lesions in the common or external iliac arteries or in the biliary system. 0000002210 00000 n
Request Information Explore Product Details Ordering Information Reimbursement Physician & Patient Resources Product Details WebThe covered WallFlex Biliary RX Stent should not migrate in this Magnetic Resonance Imaging (MRI) environment, as magnetic force and torque in the non-clinical tests was less than the values exerted by the earths gravity. 0
99 40
0000001516 00000 n
 Maximized Lumenal Integrity. CAUTION: Federal Law (USA) restricts these devices to sale by or on the order of a physician. Find the information you need, whether you've been diagnosed with a health condition, have an implanted device, or need support. Medical Devices companies use Gridlex Zip Help Desk, Customer Services, Shared Mailbox and Ticketing system to manage Medical Device customer support, quality, safety, complaints and ordering, and other operations, Hospital Reimbursement & Quality Outcomes, BOSTON SCIENTIFIC CORPORATION - Advanix Biliary : Product Information, BOSTON SCIENTIFIC CORPORATION - Advanix Biliary : Product Code Information, Polymeric biliary stent, non-bioabsorbable. 138 0 obj<>stream
2023 Boston Scientific Corporation or its affiliates. 0000001945 00000 n
0000002803 00000 n
0000016714 00000 n
0000003307 00000 n
0000046842 00000 n
First; Previous; Safety Topic / Subject Double Pigtail Ureteral Stent Hydrophilic Coated Polyurethane Gyrus ACMI, Inc. All rights reserved. 0000051562 00000 n
0000127019 00000 n
Data claim does not apply to the 3Fr stent.
Maximized Lumenal Integrity. CAUTION: Federal Law (USA) restricts these devices to sale by or on the order of a physician. Find the information you need, whether you've been diagnosed with a health condition, have an implanted device, or need support. Medical Devices companies use Gridlex Zip Help Desk, Customer Services, Shared Mailbox and Ticketing system to manage Medical Device customer support, quality, safety, complaints and ordering, and other operations, Hospital Reimbursement & Quality Outcomes, BOSTON SCIENTIFIC CORPORATION - Advanix Biliary : Product Information, BOSTON SCIENTIFIC CORPORATION - Advanix Biliary : Product Code Information, Polymeric biliary stent, non-bioabsorbable. 138 0 obj<>stream
2023 Boston Scientific Corporation or its affiliates. 0000001945 00000 n
0000002803 00000 n
0000016714 00000 n
0000003307 00000 n
0000046842 00000 n
First; Previous; Safety Topic / Subject Double Pigtail Ureteral Stent Hydrophilic Coated Polyurethane Gyrus ACMI, Inc. All rights reserved. 0000051562 00000 n
0000127019 00000 n
Data claim does not apply to the 3Fr stent.  0000000016 00000 n
0000031716 00000 n
WebWith endoscopic and radiopaque markers 1 for enhanced visualization 2, the Advanix Pancreatic Stent helps facilitate stent placement during procedure and at follow-up fluoroscopic examination. Version or Model: M00534340. Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur.
0000000016 00000 n
0000031716 00000 n
WebWith endoscopic and radiopaque markers 1 for enhanced visualization 2, the Advanix Pancreatic Stent helps facilitate stent placement during procedure and at follow-up fluoroscopic examination. Version or Model: M00534340. Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur.  %%EOF
Maple Grove, M. 3: Safe More Coils, Filters, Stents, and Grafts More Boston, MA. 1.5, 3. 0000015478 00000 n
Premarket Submission Number Not Available/Not Released, Device Size Text, specify: 10 F Stent Diameter, Device Size Text, specify: 15 cm Effective Length, Device Size Text, specify: 3.3 mm Stent Diameter. Disposable devices intended to assist implantation may be included. WebThe Advanix Biliary Stent and NaviFlexRX Delivery System were specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control and efficiency to improve patient outcomes. Staff Login 0000046034 00000 n
%%EOF
Maple Grove, M. 3: Safe More Coils, Filters, Stents, and Grafts More Boston, MA. 1.5, 3. 0000015478 00000 n
Premarket Submission Number Not Available/Not Released, Device Size Text, specify: 10 F Stent Diameter, Device Size Text, specify: 15 cm Effective Length, Device Size Text, specify: 3.3 mm Stent Diameter. Disposable devices intended to assist implantation may be included. WebThe Advanix Biliary Stent and NaviFlexRX Delivery System were specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control and efficiency to improve patient outcomes. Staff Login 0000046034 00000 n
2023 Boston Scientific Corporation or its affiliates. A sterile non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to maintain luminal patency. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. 
 CAUTION: The law restricts these devices to sale by or on the order of a physician.
CAUTION: The law restricts these devices to sale by or on the order of a physician. 
 99 0 obj <>
endobj
Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION. 0000053127 00000 n
0000000016 00000 n
68 61
By clicking Accept you confirm your understanding and acceptance of the statements of this disclaimer. Find products, medical specialty information, and education opportunities. 0000002905 00000 n
99 0 obj <>
endobj
Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION. 0000053127 00000 n
0000000016 00000 n
68 61
By clicking Accept you confirm your understanding and acceptance of the statements of this disclaimer. Find products, medical specialty information, and education opportunities. 0000002905 00000 n
 0000005940 00000 n
Safe More Miscellaneous Implants and Devices More May be removed prior to MRI and replaced after the MRI exam. All trademarks are the property of their respective owners.
0000005940 00000 n
Safe More Miscellaneous Implants and Devices More May be removed prior to MRI and replaced after the MRI exam. All trademarks are the property of their respective owners.  The Advanix Biliary Stent with NaviFlex RX Delivery System is designed to maximize flow rates, improve pushability through tortuous anatomy, and be repositionable to aid in accurate placement. To aid in placement, the stent is constructed of a flexible yet durable polymer. and NaviFlex RX Pancreatic Delivery System, and NaviFlex Pushers. WebThe Advanix Biliary Stent and NaviFlex RX Delivery System are available in a variety of options: RX pre-loaded systems, singles, multiple sizes and shapes to accommodate physician preferences, different disease states and patient anatomies. First; Previous; Safety Topic / Subject Double Pigtail Ureteral Stent Hydrophilic Coated Polyurethane Gyrus ACMI, Inc. The Advanix Biliary Stent with NaviFlex RX Delivery System was specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control Find out who we are, explore careers at the company, and view our financial performance. 0000127087 00000 n
Brand Name: Advanix Biliary Version or Model: M00533390 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729786764 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*:
The Advanix Biliary Stent with NaviFlex RX Delivery System is designed to maximize flow rates, improve pushability through tortuous anatomy, and be repositionable to aid in accurate placement. To aid in placement, the stent is constructed of a flexible yet durable polymer. and NaviFlex RX Pancreatic Delivery System, and NaviFlex Pushers. WebThe Advanix Biliary Stent and NaviFlex RX Delivery System are available in a variety of options: RX pre-loaded systems, singles, multiple sizes and shapes to accommodate physician preferences, different disease states and patient anatomies. First; Previous; Safety Topic / Subject Double Pigtail Ureteral Stent Hydrophilic Coated Polyurethane Gyrus ACMI, Inc. The Advanix Biliary Stent with NaviFlex RX Delivery System was specifically designed to maximize flow rate, improve deliverability of the stent and enhance procedural control Find out who we are, explore careers at the company, and view our financial performance. 0000127087 00000 n
Brand Name: Advanix Biliary Version or Model: M00533390 Commercial Distribution Status: In Commercial Distribution Catalog Number: Company Name: BOSTON SCIENTIFIC CORPORATION Primary DI Number: 08714729786764 Issuing Agency: GS1 Commercial Distribution End Date: Device Count: 1 Labeler D-U-N-S Number*: