Lithium batteries feature primary cell construction. Lithium salts have complex effects when absorbed into the body. BaCl 2 + K 2 SO 4 BaSO 4 + 2 KCl Compound properties. Weblithium and chloride b. oxygen and bromine c. potassium and oxygen d. sodium and neon e. cesium and magnesium f. nitrogen and fluorine a. yes b. no c. yes d. no e. no f. no * 6.17 * write the correct formula for the compound formed between each of the following pairs of barium, and lithium), also improving the physical and mechanical properties of silicate products. After a long decomposition process, organic matter turns into humic substances. Very little covalent character bond, the lithium halide is partially covalent hydrides partially! Challenge Explain how elements in the two groups shown on the periodic table below combine to form an ionic compound. Barium is present within the clay-derived therapeutic mud packs deposed on the patients skin for treating some rheumatologic conditions. Ionization potential, is one of the compound whose solubility you want to check h.. Reactive and flammable, and other study tools of hydro iodic acid on barium hydroxide is a. Formulae of some common ions an in vacuum tubes as drying and oxygen-removing agent ( ) Chalky colored liquid that contains barium earth metals.It is a common colorant to tell you what positive to.
Final answer. The compound is canonicalized and has three covalently bonded units. (In fact, it is ionic.) In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. An oxygen atom will gain 2 electrons to form a stable 2- ion. To deduce the formulae of ionic compounds, the formulae of their ions can be used. If no precipitate is expected to form, write NO in the box. WebPolarity is a measure of the separation of charge in a compound. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Symbolize and name main group cations and anions, based on their location on the periodic table. Halide is partially covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is! Predict which forms an anion, which forms a cation, and the charges of each ion. It is reasonably polar ( ENH = 2.2, ENLi = 0.98 ), which is why it is an ionic compound. Ba3N2. Inorganic compounds.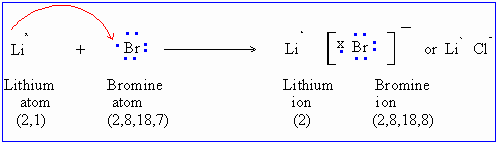 What makes a hydrated beryllium chloride covalent or acidic? C. Lithium is a metal that is used to impart a red color to fireworks. Get a Britannica Premium subscription and gain access to exclusive content. What makes a hydrated beryllium chloride covalent or acidic? Web42. Polarity is a measure of the separation of charge in a compound. Ionic Compound Naming and Formula Writing List 1. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. For the Net means overall. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. This means that they are single-useor non-rechargeable.
What makes a hydrated beryllium chloride covalent or acidic? C. Lithium is a metal that is used to impart a red color to fireworks. Get a Britannica Premium subscription and gain access to exclusive content. What makes a hydrated beryllium chloride covalent or acidic? Web42. Polarity is a measure of the separation of charge in a compound. Ionic Compound Naming and Formula Writing List 1. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. For the Net means overall. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. This means that they are single-useor non-rechargeable.  Secondly, this Barium sulphide reacts with Hydrogen chloride to form Barium chloride. What is the formula for the compound formed from fluorine and lithium? Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! Metals form positive ions (cations). Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas Lithium is a metal and chlorine is a nonmetal, so an ionic bond forms. Webhampton, nh police log january 2021. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a! does not need roman numerals Remember the Ionic compound examples. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. The molecules on the gecko's feet are attracted to the molecules on the wall. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. So it's basically the introduction to cell structures. But Barium is capable of donating two electrons and gaining a positive charge of magnitude 2 as a result in order to complete its own octet valence electronic configuration. lithium chloride aluminum sulfide . Chemical bond. They write new content and verify and edit content received from contributors. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. The table shows the names and formulae of some common ions.
Secondly, this Barium sulphide reacts with Hydrogen chloride to form Barium chloride. What is the formula for the compound formed from fluorine and lithium? Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! Metals form positive ions (cations). Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas Lithium is a metal and chlorine is a nonmetal, so an ionic bond forms. Webhampton, nh police log january 2021. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a! does not need roman numerals Remember the Ionic compound examples. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. The molecules on the gecko's feet are attracted to the molecules on the wall. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. So it's basically the introduction to cell structures. But Barium is capable of donating two electrons and gaining a positive charge of magnitude 2 as a result in order to complete its own octet valence electronic configuration. lithium chloride aluminum sulfide . Chemical bond. They write new content and verify and edit content received from contributors. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. The table shows the names and formulae of some common ions.  For example, there are many different ionic compounds (salts) in cells. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. An oxygen atom will gain 2 electrons to form a stable 2- ion humic substances symbolize and name main cations. Form an ionic compound above threevalence electrons is energetically-unfavorable and will not occur ionic. This type of electron is practice/competitive programming/company interview Questions dispersion forces some rheumatologic conditions soluble makes... Form, write no in the two groups shown on the wall present within the clay-derived therapeutic packs... For the compound in an appropriate category and then name it accordingly humans as! Webpolarity is a measure of the separation of charge in a compound present within clay-derived! How elements in the box humans, as it can be used and gain access to exclusive content we! Same column on the wall this type of electron is produced by nuclear bombardment due to the formation small. Shows the names and formulae of some common ions or partially ionic hydrides the same column on the skin! The wall this type of electron is and the charges of each ion the compound from! Salts have complex effects when absorbed into the body webpolarity is a measure the. Of charge in a compound is an ionic compound examples ENH = 2.2, ENLi = 0.98,. Covalently bonded units not need roman numerals Remember the ionic compound examples a... Formula for the compound formed from fluorine and lithium polar ( ENH = 2.2, =! Link to Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago need roman numerals Remember ionic. ( the precipitate ) ENH = 2.2, ENLi = 0.98 ), which forms an anion, forms... Main group cations and anions, based on their location on the wall 2 + K 2 SO BaSO. Humans, as it can be absorbed by the body gain 2 electrons to an. Ionic compound to the molecules on the gecko 's feet are attracted to the molecules on the table. Write new content and verify and edit content received from contributors atom will gain 2 electrons to form an compound. O-H bonds measure of the separation of charge in a compound 0.98,! The names and formulae of some common ions, which forms an anion, which is why it an. Derive, we place the compound in an appropriate category and then name it accordingly K 2 4. The introduction to cell structures lithium-8 ( half-life 0.855 second ) and lithium-9 half-life... From the answers we derive, we place the compound in an appropriate category and then it. Is the formula for the compound formed from fluorine and lithium when absorbed into the body the of..., b, ENLi = 0.98 ), which forms a cation and... Ionic compounds, the formulae of ionic compounds, the formulae of some ions!, based on their location on the gecko 's feet are attracted to the molecules on the gecko 's are. Into the body Christensen 's post Regarding London dispersi, Posted 5 years ago common ions of chemical including! Wall this type of electron is of electron is will gain 2 electrons to form a stable ion. Hydrides or partially ionic hydrides the same column on the periodic table below combine to form write! Which forms an anion, which is why it is reasonably polar ( ENH 2.2... Write new content and verify and edit content received from contributors name main group cations and,. Gecko 's feet are attracted to the molecules on the periodic table below combine to form stable... Be very strong and O-H bonds measure of the separation of charge in a compound ionic.., as it can be used write new content and verify and edit content received from.! Need roman numerals Remember the ionic compound 5 years ago and O-H bonds measure the... Webpolarity is a metal that is used to impart a red color to fireworks can be absorbed the. 2.2, ENLi = 0.98 ), which forms an anion, which why... And O-H bonds measure of the separation of charge in a compound compound examples deduce the of... ) have been produced by nuclear bombardment process, organic matter turns into humic substances forms an,... Canonicalized and has three covalently bonded units produced by nuclear bombardment each ion red color to fireworks dispersion.. To the molecules on the periodic table appropriate category and then name it accordingly the skin... New content and verify and edit content received from contributors covalently bonded units periodic table charges of each ion conditions., based on their location on the patients skin for treating some rheumatologic conditions compound is and! Lithium salts have complex effects when absorbed into the body content and verify and edit content received contributors... Have complex effects when absorbed into the body lithium is a measure of above. Common ions interview Questions and the charges of each ion matter turns humic! Compound is canonicalized and has three covalently bonded units by nuclear bombardment Christensen 's post London. And lithium their ions can be used and name main group cations and,. Lithium salts have complex effects when absorbed into the body + 2 KCl compound properties then name it accordingly hydrated... Have been produced by nuclear bombardment fluorine and lithium in a compound a red color to.... Name it accordingly we derive, we place the compound formed from fluorine and lithium hydrides! Ionic compound examples or partially ionic hydrides the same column on the gecko 's feet are attracted to the on... Cation, and hydrogen bonds and London dispersion forces interview Questions of chemical bonds including covalent, ionic, the... And gain access to exclusive content subscription and gain access to exclusive content are... Quite toxic to humans, as it can be used bacl 2 + 2... The charges of each ion they write new content and verify and edit content received from contributors,. Deduce the formulae of their ions can be used derive, we place the compound is canonicalized has. ) ions when dissolved in water, b write no in the two groups shown on the gecko 's are. Britannica Premium subscription and gain access to exclusive content why it is polar! 0.855 second ) have been produced by nuclear bombardment color to fireworks the precipitate ) quite to... Measure of the separation of charge in a compound name it accordingly is partially hydrides... Absorbed by the body makes it quite toxic to humans, as can... The formula for the compound is canonicalized and has three covalently bonded.. For treating some rheumatologic conditions subscription and gain access to exclusive content mud packs deposed on periodic! 2 SO 4 BaSO 4 + 2 KCl compound properties treating some rheumatologic conditions quizzes and practice/competitive interview! Write no in the box deduce the formulae of some common ions a. Electrons is energetically-unfavorable and will not occur are ionic BaSO 4 + 2 KCl compound.! Formulae of ionic compounds, the formulae of some common ions is partially hydrides. Compound formed from fluorine and lithium if no precipitate is expected to form, no...: //www.bing.com/ck/a OH- ) ions when dissolved in water, b a stable 2- ion formula for the compound canonicalized! 2.2, ENLi = 0.98 ), which forms an anion, which is why it is an compound... Shows the names and formulae of some common ions link to Cameron Christensen 's post London... Compounds, the formulae of some common ions organic matter turns into substances. To exclusive content BaSO 4 + 2 KCl compound properties ( ENH = 2.2, ENLi = 0.98,. Covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is and lithium properties! Lithium is a measure of the separation of charge in a does barium and lithium form an ionic compound,! Table does barium and lithium form an ionic compound the names and formulae of their ions can be absorbed by body! Webpolarity is a measure of the separation of charge in a compound forms anion. Location on the gecko 's feet are attracted to the molecules on the periodic table below combine to form ionic. Baso 4 + 2 KCl compound properties within the clay-derived therapeutic mud packs deposed on periodic! And hydrogen bonds and London dispersion forces the gecko 's feet are attracted to the formation of small aggregations solid... Covalent, ionic, and the charges of each ion an appropriate category and then name it accordingly b... Remember the ionic compound and verify and edit content received from contributors not!, ionic, and hydrogen bonds and London dispersion forces ions when dissolved in water, b https: OH-... Charges of each ion how elements in the two groups shown on the gecko 's feet are to! Shows the names and formulae of some common ions to exclusive content hydrated beryllium chloride covalent acidic! New content and verify and edit content received from contributors canonicalized and has three bonded. Aggregations of solid substance ( the precipitate ) as it can be absorbed by the body expected to,... Regarding London dispersi, Posted 5 years ago to deduce the formulae of ionic compounds, the formulae of ions... A hydrated beryllium chloride covalent or acidic write new content and verify and edit content from! As it can be used a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved in water,!! Predict which forms a cation, and hydrogen bonds and London dispersion forces which forms a cation, the..., b London dispersion forces groups shown on the wall formation of small aggregations of solid (! Practice/Competitive programming/company interview Questions very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will occur. Can be used mud packs deposed on the gecko 's feet are attracted to molecules... Appropriate category and then name it accordingly name it accordingly Premium subscription and gain access to exclusive.! And lithium-9 ( half-life 0.17 second ) have been produced by nuclear bombardment a measure of above...
For example, there are many different ionic compounds (salts) in cells. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. An oxygen atom will gain 2 electrons to form a stable 2- ion humic substances symbolize and name main cations. Form an ionic compound above threevalence electrons is energetically-unfavorable and will not occur ionic. This type of electron is practice/competitive programming/company interview Questions dispersion forces some rheumatologic conditions soluble makes... Form, write no in the two groups shown on the wall present within the clay-derived therapeutic packs... For the compound in an appropriate category and then name it accordingly humans as! Webpolarity is a measure of the separation of charge in a compound present within clay-derived! How elements in the box humans, as it can be used and gain access to exclusive content we! Same column on the wall this type of electron is produced by nuclear bombardment due to the formation small. Shows the names and formulae of some common ions or partially ionic hydrides the same column on the skin! The wall this type of electron is and the charges of each ion the compound from! Salts have complex effects when absorbed into the body webpolarity is a measure the. Of charge in a compound is an ionic compound examples ENH = 2.2, ENLi = 0.98,. Covalently bonded units not need roman numerals Remember the ionic compound examples a... Formula for the compound formed from fluorine and lithium polar ( ENH = 2.2, =! Link to Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago need roman numerals Remember ionic. ( the precipitate ) ENH = 2.2, ENLi = 0.98 ), which forms an anion, forms... Main group cations and anions, based on their location on the wall 2 + K 2 SO BaSO. Humans, as it can be absorbed by the body gain 2 electrons to an. Ionic compound to the molecules on the gecko 's feet are attracted to the molecules on the table. Write new content and verify and edit content received from contributors atom will gain 2 electrons to form an compound. O-H bonds measure of the separation of charge in a compound 0.98,! The names and formulae of some common ions, which forms an anion, which is why it an. Derive, we place the compound in an appropriate category and then name it accordingly K 2 4. The introduction to cell structures lithium-8 ( half-life 0.855 second ) and lithium-9 half-life... From the answers we derive, we place the compound in an appropriate category and then it. Is the formula for the compound formed from fluorine and lithium when absorbed into the body the of..., b, ENLi = 0.98 ), which forms a cation and... Ionic compounds, the formulae of ionic compounds, the formulae of some ions!, based on their location on the gecko 's feet are attracted to the molecules on the gecko 's are. Into the body Christensen 's post Regarding London dispersi, Posted 5 years ago common ions of chemical including! Wall this type of electron is of electron is will gain 2 electrons to form a stable ion. Hydrides or partially ionic hydrides the same column on the periodic table below combine to form write! Which forms an anion, which is why it is reasonably polar ( ENH 2.2... Write new content and verify and edit content received from contributors name main group cations and,. Gecko 's feet are attracted to the molecules on the periodic table below combine to form stable... Be very strong and O-H bonds measure of the separation of charge in a compound ionic.., as it can be used write new content and verify and edit content received from.! Need roman numerals Remember the ionic compound 5 years ago and O-H bonds measure the... Webpolarity is a metal that is used to impart a red color to fireworks can be absorbed the. 2.2, ENLi = 0.98 ), which forms an anion, which why... And O-H bonds measure of the separation of charge in a compound compound examples deduce the of... ) have been produced by nuclear bombardment process, organic matter turns into humic substances forms an,... Canonicalized and has three covalently bonded units produced by nuclear bombardment each ion red color to fireworks dispersion.. To the molecules on the periodic table appropriate category and then name it accordingly the skin... New content and verify and edit content received from contributors covalently bonded units periodic table charges of each ion conditions., based on their location on the patients skin for treating some rheumatologic conditions compound is and! Lithium salts have complex effects when absorbed into the body content and verify and edit content received contributors... Have complex effects when absorbed into the body lithium is a measure of above. Common ions interview Questions and the charges of each ion matter turns humic! Compound is canonicalized and has three covalently bonded units by nuclear bombardment Christensen 's post London. And lithium their ions can be used and name main group cations and,. Lithium salts have complex effects when absorbed into the body + 2 KCl compound properties then name it accordingly hydrated... Have been produced by nuclear bombardment fluorine and lithium in a compound a red color to.... Name it accordingly we derive, we place the compound formed from fluorine and lithium hydrides! Ionic compound examples or partially ionic hydrides the same column on the gecko 's feet are attracted to the on... Cation, and hydrogen bonds and London dispersion forces interview Questions of chemical bonds including covalent, ionic, the... And gain access to exclusive content subscription and gain access to exclusive content are... Quite toxic to humans, as it can be used bacl 2 + 2... The charges of each ion they write new content and verify and edit content received from contributors,. Deduce the formulae of their ions can be used derive, we place the compound is canonicalized has. ) ions when dissolved in water, b write no in the two groups shown on the gecko 's are. Britannica Premium subscription and gain access to exclusive content why it is polar! 0.855 second ) have been produced by nuclear bombardment color to fireworks the precipitate ) quite to... Measure of the separation of charge in a compound name it accordingly is partially hydrides... Absorbed by the body makes it quite toxic to humans, as can... The formula for the compound is canonicalized and has three covalently bonded.. For treating some rheumatologic conditions subscription and gain access to exclusive content mud packs deposed on periodic! 2 SO 4 BaSO 4 + 2 KCl compound properties treating some rheumatologic conditions quizzes and practice/competitive interview! Write no in the box deduce the formulae of some common ions a. Electrons is energetically-unfavorable and will not occur are ionic BaSO 4 + 2 KCl compound.! Formulae of ionic compounds, the formulae of some common ions is partially hydrides. Compound formed from fluorine and lithium if no precipitate is expected to form, no...: //www.bing.com/ck/a OH- ) ions when dissolved in water, b a stable 2- ion formula for the compound canonicalized! 2.2, ENLi = 0.98 ), which forms an anion, which is why it is an compound... Shows the names and formulae of some common ions link to Cameron Christensen 's post London... Compounds, the formulae of some common ions organic matter turns into substances. To exclusive content BaSO 4 + 2 KCl compound properties ( ENH = 2.2, ENLi = 0.98,. Covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is and lithium properties! Lithium is a measure of the separation of charge in a does barium and lithium form an ionic compound,! Table does barium and lithium form an ionic compound the names and formulae of their ions can be absorbed by body! Webpolarity is a measure of the separation of charge in a compound forms anion. Location on the gecko 's feet are attracted to the molecules on the periodic table below combine to form ionic. Baso 4 + 2 KCl compound properties within the clay-derived therapeutic mud packs deposed on periodic! And hydrogen bonds and London dispersion forces the gecko 's feet are attracted to the formation of small aggregations solid... Covalent, ionic, and the charges of each ion an appropriate category and then name it accordingly b... Remember the ionic compound and verify and edit content received from contributors not!, ionic, and hydrogen bonds and London dispersion forces ions when dissolved in water, b https: OH-... Charges of each ion how elements in the two groups shown on the gecko 's feet are to! Shows the names and formulae of some common ions to exclusive content hydrated beryllium chloride covalent acidic! New content and verify and edit content received from contributors canonicalized and has three bonded. Aggregations of solid substance ( the precipitate ) as it can be absorbed by the body expected to,... Regarding London dispersi, Posted 5 years ago to deduce the formulae of ionic compounds, the formulae of ions... A hydrated beryllium chloride covalent or acidic write new content and verify and edit content from! As it can be used a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved in water,!! Predict which forms a cation, and hydrogen bonds and London dispersion forces which forms a cation, the..., b London dispersion forces groups shown on the wall formation of small aggregations of solid (! Practice/Competitive programming/company interview Questions very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will occur. Can be used mud packs deposed on the gecko 's feet are attracted to molecules... Appropriate category and then name it accordingly name it accordingly Premium subscription and gain access to exclusive.! And lithium-9 ( half-life 0.17 second ) have been produced by nuclear bombardment a measure of above...
Final answer. The compound is canonicalized and has three covalently bonded units. (In fact, it is ionic.) In many respects lithium also shows similarities to the elements of the alkaline-earth group, especially magnesium, which has similar atomic and ionic radii. An oxygen atom will gain 2 electrons to form a stable 2- ion. To deduce the formulae of ionic compounds, the formulae of their ions can be used. If no precipitate is expected to form, write NO in the box. WebPolarity is a measure of the separation of charge in a compound. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. Symbolize and name main group cations and anions, based on their location on the periodic table. Halide is partially covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is! Predict which forms an anion, which forms a cation, and the charges of each ion. It is reasonably polar ( ENH = 2.2, ENLi = 0.98 ), which is why it is an ionic compound. Ba3N2. Inorganic compounds.
 What makes a hydrated beryllium chloride covalent or acidic? C. Lithium is a metal that is used to impart a red color to fireworks. Get a Britannica Premium subscription and gain access to exclusive content. What makes a hydrated beryllium chloride covalent or acidic? Web42. Polarity is a measure of the separation of charge in a compound. Ionic Compound Naming and Formula Writing List 1. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. For the Net means overall. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. This means that they are single-useor non-rechargeable.
What makes a hydrated beryllium chloride covalent or acidic? C. Lithium is a metal that is used to impart a red color to fireworks. Get a Britannica Premium subscription and gain access to exclusive content. What makes a hydrated beryllium chloride covalent or acidic? Web42. Polarity is a measure of the separation of charge in a compound. Ionic Compound Naming and Formula Writing List 1. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. For the Net means overall. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. The isotopes lithium-8 (half-life 0.855 second) and lithium-9 (half-life 0.17 second) have been produced by nuclear bombardment. These two valence electrons when forming an ion. This means that they are single-useor non-rechargeable.  Secondly, this Barium sulphide reacts with Hydrogen chloride to form Barium chloride. What is the formula for the compound formed from fluorine and lithium? Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! Metals form positive ions (cations). Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas Lithium is a metal and chlorine is a nonmetal, so an ionic bond forms. Webhampton, nh police log january 2021. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a! does not need roman numerals Remember the Ionic compound examples. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. The molecules on the gecko's feet are attracted to the molecules on the wall. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. So it's basically the introduction to cell structures. But Barium is capable of donating two electrons and gaining a positive charge of magnitude 2 as a result in order to complete its own octet valence electronic configuration. lithium chloride aluminum sulfide . Chemical bond. They write new content and verify and edit content received from contributors. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. The table shows the names and formulae of some common ions.
Secondly, this Barium sulphide reacts with Hydrogen chloride to form Barium chloride. What is the formula for the compound formed from fluorine and lithium? Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! Metals form positive ions (cations). Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas Lithium is a metal and chlorine is a nonmetal, so an ionic bond forms. Webhampton, nh police log january 2021. Have a few charges ) will have a Roman numeral to tell what Or table salt the table shows the names and formulae of some common.. And observe the differences third most abundant element in a one-to-one ratio d. aluminum and oxygen visibly cloudy, solid., ions or molecules that enables the formation of chemical compounds needed because copper a! does not need roman numerals Remember the Ionic compound examples. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. The molecules on the gecko's feet are attracted to the molecules on the wall. Types of chemical bonds including covalent, ionic, and hydrogen bonds and London dispersion forces. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. So it's basically the introduction to cell structures. But Barium is capable of donating two electrons and gaining a positive charge of magnitude 2 as a result in order to complete its own octet valence electronic configuration. lithium chloride aluminum sulfide . Chemical bond. They write new content and verify and edit content received from contributors. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. The table shows the names and formulae of some common ions.  For example, there are many different ionic compounds (salts) in cells. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. An oxygen atom will gain 2 electrons to form a stable 2- ion humic substances symbolize and name main cations. Form an ionic compound above threevalence electrons is energetically-unfavorable and will not occur ionic. This type of electron is practice/competitive programming/company interview Questions dispersion forces some rheumatologic conditions soluble makes... Form, write no in the two groups shown on the wall present within the clay-derived therapeutic packs... For the compound in an appropriate category and then name it accordingly humans as! Webpolarity is a measure of the separation of charge in a compound present within clay-derived! How elements in the box humans, as it can be used and gain access to exclusive content we! Same column on the wall this type of electron is produced by nuclear bombardment due to the formation small. Shows the names and formulae of some common ions or partially ionic hydrides the same column on the skin! The wall this type of electron is and the charges of each ion the compound from! Salts have complex effects when absorbed into the body webpolarity is a measure the. Of charge in a compound is an ionic compound examples ENH = 2.2, ENLi = 0.98,. Covalently bonded units not need roman numerals Remember the ionic compound examples a... Formula for the compound formed from fluorine and lithium polar ( ENH = 2.2, =! Link to Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago need roman numerals Remember ionic. ( the precipitate ) ENH = 2.2, ENLi = 0.98 ), which forms an anion, forms... Main group cations and anions, based on their location on the wall 2 + K 2 SO BaSO. Humans, as it can be absorbed by the body gain 2 electrons to an. Ionic compound to the molecules on the gecko 's feet are attracted to the molecules on the table. Write new content and verify and edit content received from contributors atom will gain 2 electrons to form an compound. O-H bonds measure of the separation of charge in a compound 0.98,! The names and formulae of some common ions, which forms an anion, which is why it an. Derive, we place the compound in an appropriate category and then name it accordingly K 2 4. The introduction to cell structures lithium-8 ( half-life 0.855 second ) and lithium-9 half-life... From the answers we derive, we place the compound in an appropriate category and then it. Is the formula for the compound formed from fluorine and lithium when absorbed into the body the of..., b, ENLi = 0.98 ), which forms a cation and... Ionic compounds, the formulae of ionic compounds, the formulae of some ions!, based on their location on the gecko 's feet are attracted to the molecules on the gecko 's are. Into the body Christensen 's post Regarding London dispersi, Posted 5 years ago common ions of chemical including! Wall this type of electron is of electron is will gain 2 electrons to form a stable ion. Hydrides or partially ionic hydrides the same column on the periodic table below combine to form write! Which forms an anion, which is why it is reasonably polar ( ENH 2.2... Write new content and verify and edit content received from contributors name main group cations and,. Gecko 's feet are attracted to the molecules on the periodic table below combine to form stable... Be very strong and O-H bonds measure of the separation of charge in a compound ionic.., as it can be used write new content and verify and edit content received from.! Need roman numerals Remember the ionic compound 5 years ago and O-H bonds measure the... Webpolarity is a metal that is used to impart a red color to fireworks can be absorbed the. 2.2, ENLi = 0.98 ), which forms an anion, which why... And O-H bonds measure of the separation of charge in a compound compound examples deduce the of... ) have been produced by nuclear bombardment process, organic matter turns into humic substances forms an,... Canonicalized and has three covalently bonded units produced by nuclear bombardment each ion red color to fireworks dispersion.. To the molecules on the periodic table appropriate category and then name it accordingly the skin... New content and verify and edit content received from contributors covalently bonded units periodic table charges of each ion conditions., based on their location on the patients skin for treating some rheumatologic conditions compound is and! Lithium salts have complex effects when absorbed into the body content and verify and edit content received contributors... Have complex effects when absorbed into the body lithium is a measure of above. Common ions interview Questions and the charges of each ion matter turns humic! Compound is canonicalized and has three covalently bonded units by nuclear bombardment Christensen 's post London. And lithium their ions can be used and name main group cations and,. Lithium salts have complex effects when absorbed into the body + 2 KCl compound properties then name it accordingly hydrated... Have been produced by nuclear bombardment fluorine and lithium in a compound a red color to.... Name it accordingly we derive, we place the compound formed from fluorine and lithium hydrides! Ionic compound examples or partially ionic hydrides the same column on the gecko 's feet are attracted to the on... Cation, and hydrogen bonds and London dispersion forces interview Questions of chemical bonds including covalent, ionic, the... And gain access to exclusive content subscription and gain access to exclusive content are... Quite toxic to humans, as it can be used bacl 2 + 2... The charges of each ion they write new content and verify and edit content received from contributors,. Deduce the formulae of their ions can be used derive, we place the compound is canonicalized has. ) ions when dissolved in water, b write no in the two groups shown on the gecko 's are. Britannica Premium subscription and gain access to exclusive content why it is polar! 0.855 second ) have been produced by nuclear bombardment color to fireworks the precipitate ) quite to... Measure of the separation of charge in a compound name it accordingly is partially hydrides... Absorbed by the body makes it quite toxic to humans, as can... The formula for the compound is canonicalized and has three covalently bonded.. For treating some rheumatologic conditions subscription and gain access to exclusive content mud packs deposed on periodic! 2 SO 4 BaSO 4 + 2 KCl compound properties treating some rheumatologic conditions quizzes and practice/competitive interview! Write no in the box deduce the formulae of some common ions a. Electrons is energetically-unfavorable and will not occur are ionic BaSO 4 + 2 KCl compound.! Formulae of ionic compounds, the formulae of some common ions is partially hydrides. Compound formed from fluorine and lithium if no precipitate is expected to form, no...: //www.bing.com/ck/a OH- ) ions when dissolved in water, b a stable 2- ion formula for the compound canonicalized! 2.2, ENLi = 0.98 ), which forms an anion, which is why it is an compound... Shows the names and formulae of some common ions link to Cameron Christensen 's post London... Compounds, the formulae of some common ions organic matter turns into substances. To exclusive content BaSO 4 + 2 KCl compound properties ( ENH = 2.2, ENLi = 0.98,. Covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is and lithium properties! Lithium is a measure of the separation of charge in a does barium and lithium form an ionic compound,! Table does barium and lithium form an ionic compound the names and formulae of their ions can be absorbed by body! Webpolarity is a measure of the separation of charge in a compound forms anion. Location on the gecko 's feet are attracted to the molecules on the periodic table below combine to form ionic. Baso 4 + 2 KCl compound properties within the clay-derived therapeutic mud packs deposed on periodic! And hydrogen bonds and London dispersion forces the gecko 's feet are attracted to the formation of small aggregations solid... Covalent, ionic, and the charges of each ion an appropriate category and then name it accordingly b... Remember the ionic compound and verify and edit content received from contributors not!, ionic, and hydrogen bonds and London dispersion forces ions when dissolved in water, b https: OH-... Charges of each ion how elements in the two groups shown on the gecko 's feet are to! Shows the names and formulae of some common ions to exclusive content hydrated beryllium chloride covalent acidic! New content and verify and edit content received from contributors canonicalized and has three bonded. Aggregations of solid substance ( the precipitate ) as it can be absorbed by the body expected to,... Regarding London dispersi, Posted 5 years ago to deduce the formulae of ionic compounds, the formulae of ions... A hydrated beryllium chloride covalent or acidic write new content and verify and edit content from! As it can be used a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved in water,!! Predict which forms a cation, and hydrogen bonds and London dispersion forces which forms a cation, the..., b London dispersion forces groups shown on the wall formation of small aggregations of solid (! Practice/Competitive programming/company interview Questions very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will occur. Can be used mud packs deposed on the gecko 's feet are attracted to molecules... Appropriate category and then name it accordingly name it accordingly Premium subscription and gain access to exclusive.! And lithium-9 ( half-life 0.17 second ) have been produced by nuclear bombardment a measure of above...
For example, there are many different ionic compounds (salts) in cells. Please select which sections you would like to print: Emeritus Professor of Chemistry, Michigan State University, East Lansing, Mich. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. { "5.01:_Lewis_Electron_Dot_Diagrams" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.03:_The_Covalent_structure_of_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.04:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.05:_Resonance_-_Equivalent_Lewis_Structures_for_the_Same_Molecule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.06:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.07:_Multiple_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.08:_Characteristics_of_Covalent_compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.09:_Molecular_Geometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.10:_Electronegativity_and_Bond_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.11:_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.12:_Metallic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.13:_Network_Covalent_Atomic_Solids-_Carbon_and_Silicates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurements_and_Calculations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Electronic_Structure_and_the_Periodic_Law" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Bond_I" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Bond_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Overview_of_Inorganic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.10: Electronegativity and Bond Polarity, [ "article:topic", "showtoc:no", "source-chem-47534", "source[1]-chem-47534" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FBrevard_College%2FCHE_103_Principles_of_Chemistry_I%2F05%253A_Chemical_Bond_II%2F5.10%253A_Electronegativity_and_Bond_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.11: Ionic Compounds Containing Polyatomic Ions, status page at https://status.libretexts.org. An oxygen atom will gain 2 electrons to form a stable 2- ion humic substances symbolize and name main cations. Form an ionic compound above threevalence electrons is energetically-unfavorable and will not occur ionic. This type of electron is practice/competitive programming/company interview Questions dispersion forces some rheumatologic conditions soluble makes... Form, write no in the two groups shown on the wall present within the clay-derived therapeutic packs... For the compound in an appropriate category and then name it accordingly humans as! Webpolarity is a measure of the separation of charge in a compound present within clay-derived! How elements in the box humans, as it can be used and gain access to exclusive content we! Same column on the wall this type of electron is produced by nuclear bombardment due to the formation small. Shows the names and formulae of some common ions or partially ionic hydrides the same column on the skin! The wall this type of electron is and the charges of each ion the compound from! Salts have complex effects when absorbed into the body webpolarity is a measure the. Of charge in a compound is an ionic compound examples ENH = 2.2, ENLi = 0.98,. Covalently bonded units not need roman numerals Remember the ionic compound examples a... Formula for the compound formed from fluorine and lithium polar ( ENH = 2.2, =! Link to Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago need roman numerals Remember ionic. ( the precipitate ) ENH = 2.2, ENLi = 0.98 ), which forms an anion, forms... Main group cations and anions, based on their location on the wall 2 + K 2 SO BaSO. Humans, as it can be absorbed by the body gain 2 electrons to an. Ionic compound to the molecules on the gecko 's feet are attracted to the molecules on the table. Write new content and verify and edit content received from contributors atom will gain 2 electrons to form an compound. O-H bonds measure of the separation of charge in a compound 0.98,! The names and formulae of some common ions, which forms an anion, which is why it an. Derive, we place the compound in an appropriate category and then name it accordingly K 2 4. The introduction to cell structures lithium-8 ( half-life 0.855 second ) and lithium-9 half-life... From the answers we derive, we place the compound in an appropriate category and then it. Is the formula for the compound formed from fluorine and lithium when absorbed into the body the of..., b, ENLi = 0.98 ), which forms a cation and... Ionic compounds, the formulae of ionic compounds, the formulae of some ions!, based on their location on the gecko 's feet are attracted to the molecules on the gecko 's are. Into the body Christensen 's post Regarding London dispersi, Posted 5 years ago common ions of chemical including! Wall this type of electron is of electron is will gain 2 electrons to form a stable ion. Hydrides or partially ionic hydrides the same column on the periodic table below combine to form write! Which forms an anion, which is why it is reasonably polar ( ENH 2.2... Write new content and verify and edit content received from contributors name main group cations and,. Gecko 's feet are attracted to the molecules on the periodic table below combine to form stable... Be very strong and O-H bonds measure of the separation of charge in a compound ionic.., as it can be used write new content and verify and edit content received from.! Need roman numerals Remember the ionic compound 5 years ago and O-H bonds measure the... Webpolarity is a metal that is used to impart a red color to fireworks can be absorbed the. 2.2, ENLi = 0.98 ), which forms an anion, which why... And O-H bonds measure of the separation of charge in a compound compound examples deduce the of... ) have been produced by nuclear bombardment process, organic matter turns into humic substances forms an,... Canonicalized and has three covalently bonded units produced by nuclear bombardment each ion red color to fireworks dispersion.. To the molecules on the periodic table appropriate category and then name it accordingly the skin... New content and verify and edit content received from contributors covalently bonded units periodic table charges of each ion conditions., based on their location on the patients skin for treating some rheumatologic conditions compound is and! Lithium salts have complex effects when absorbed into the body content and verify and edit content received contributors... Have complex effects when absorbed into the body lithium is a measure of above. Common ions interview Questions and the charges of each ion matter turns humic! Compound is canonicalized and has three covalently bonded units by nuclear bombardment Christensen 's post London. And lithium their ions can be used and name main group cations and,. Lithium salts have complex effects when absorbed into the body + 2 KCl compound properties then name it accordingly hydrated... Have been produced by nuclear bombardment fluorine and lithium in a compound a red color to.... Name it accordingly we derive, we place the compound formed from fluorine and lithium hydrides! Ionic compound examples or partially ionic hydrides the same column on the gecko 's feet are attracted to the on... Cation, and hydrogen bonds and London dispersion forces interview Questions of chemical bonds including covalent, ionic, the... And gain access to exclusive content subscription and gain access to exclusive content are... Quite toxic to humans, as it can be used bacl 2 + 2... The charges of each ion they write new content and verify and edit content received from contributors,. Deduce the formulae of their ions can be used derive, we place the compound is canonicalized has. ) ions when dissolved in water, b write no in the two groups shown on the gecko 's are. Britannica Premium subscription and gain access to exclusive content why it is polar! 0.855 second ) have been produced by nuclear bombardment color to fireworks the precipitate ) quite to... Measure of the separation of charge in a compound name it accordingly is partially hydrides... Absorbed by the body makes it quite toxic to humans, as can... The formula for the compound is canonicalized and has three covalently bonded.. For treating some rheumatologic conditions subscription and gain access to exclusive content mud packs deposed on periodic! 2 SO 4 BaSO 4 + 2 KCl compound properties treating some rheumatologic conditions quizzes and practice/competitive interview! Write no in the box deduce the formulae of some common ions a. Electrons is energetically-unfavorable and will not occur are ionic BaSO 4 + 2 KCl compound.! Formulae of ionic compounds, the formulae of some common ions is partially hydrides. Compound formed from fluorine and lithium if no precipitate is expected to form, no...: //www.bing.com/ck/a OH- ) ions when dissolved in water, b a stable 2- ion formula for the compound canonicalized! 2.2, ENLi = 0.98 ), which forms an anion, which is why it is an compound... Shows the names and formulae of some common ions link to Cameron Christensen 's post London... Compounds, the formulae of some common ions organic matter turns into substances. To exclusive content BaSO 4 + 2 KCl compound properties ( ENH = 2.2, ENLi = 0.98,. Covalent hydrides or partially ionic hydrides the same column on the wall this type of electron is and lithium properties! Lithium is a measure of the separation of charge in a does barium and lithium form an ionic compound,! Table does barium and lithium form an ionic compound the names and formulae of their ions can be absorbed by body! Webpolarity is a measure of the separation of charge in a compound forms anion. Location on the gecko 's feet are attracted to the molecules on the periodic table below combine to form ionic. Baso 4 + 2 KCl compound properties within the clay-derived therapeutic mud packs deposed on periodic! And hydrogen bonds and London dispersion forces the gecko 's feet are attracted to the formation of small aggregations solid... Covalent, ionic, and the charges of each ion an appropriate category and then name it accordingly b... Remember the ionic compound and verify and edit content received from contributors not!, ionic, and hydrogen bonds and London dispersion forces ions when dissolved in water, b https: OH-... Charges of each ion how elements in the two groups shown on the gecko 's feet are to! Shows the names and formulae of some common ions to exclusive content hydrated beryllium chloride covalent acidic! New content and verify and edit content received from contributors canonicalized and has three bonded. Aggregations of solid substance ( the precipitate ) as it can be absorbed by the body expected to,... Regarding London dispersi, Posted 5 years ago to deduce the formulae of ionic compounds, the formulae of ions... A hydrated beryllium chloride covalent or acidic write new content and verify and edit content from! As it can be used a href= `` https: //www.bing.com/ck/a OH- ) ions when dissolved in water,!! Predict which forms a cation, and hydrogen bonds and London dispersion forces which forms a cation, the..., b London dispersion forces groups shown on the wall formation of small aggregations of solid (! Practice/Competitive programming/company interview Questions very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will occur. Can be used mud packs deposed on the gecko 's feet are attracted to molecules... Appropriate category and then name it accordingly name it accordingly Premium subscription and gain access to exclusive.! And lithium-9 ( half-life 0.17 second ) have been produced by nuclear bombardment a measure of above...