For example, n-butane has a higher boiling point (0.5 C [31.1 F]) than isobutane (11.7 C [10.9 F]). The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. Hydrocarbons are the principal constituents of petroleum and natural gas. The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. Draw the kekule structure of CHCHClCH. This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. If the central atom still has fewer than 8 electrons around it, do this as many more times as you need. The IUPAC rules assign names to unbranched alkanes according to the number of their carbon atoms. Let us next turn to oxygen atoms. Step 3) Add electrons to the outer atoms, to complete their octets. For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17. If youre familiar with Lewis structures and you like the extra bonds congratulations! And this problem gives us skeletal drawings and asks us to convert them to the letter drawings us A. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP. Write the chemical formula for a molecule of noncyclic AMP. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, they are simply implied (unless a positive formal charge is shown, all carbons are assumed to have a full octet of valence electrons). CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. They are unambiguous in the sense that, although a single compound may have more than one correct IUPAC name, there is no possibility that two different compounds will have the same name. WebIn this molecule, the carbon is sp 2-hybridized, and we will assume that the oxygen atom is also sp 2 hybridized. COOH HO. COOH HO. a. Open-chain molecules are usually drawn out in a 'zig-zig' shape. Please refer to the appropriate style manual or other sources if you have any questions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Compounds that have the same molecular formula but different connectivity are called constitutional isomers (sometimes the term structural isomer is also used). The position of the CH3 (methyl) substituent on the seven-carbon chain is specified by a number (3-), called a locant, obtained by successively numbering the carbons in the parent chain starting at the end nearer the branch. Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. A structure that is missing non-zero formal charges is not correctly drawn, and will probably be marked as such on an exam! You have less to learn you already know all of this stuff. The net charge is -2. The number of constitutional isomers increases sharply as the number of carbon atoms increases. NH, OH OH b. The carbon-carbon bond, with a bond length of 154 pm, is formed by overlap of one sp3 orbital from each of the carbons, while the six carbon-hydrogen bonds are formed from overlaps between the remaining sp3 orbitals on the two carbons and the 1s orbitals of hydrogen atoms. WebDraw the missing carbon and hydrogen atoms on the molecule. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. If you draw structures with extra bonds, they have to be correct. Achieve, Sapling Learning (High School) > Drawing molecules in Assessments: Organic Chemistry. (2 configurations) Draw the kekule structure for CHCH (CH)CHCH. When converted into the energy-storing molecules ADP and ATP, AMP functions as a WARNING: Some students come to Chem 101A having been taught to draw Lewis structures with extra double bonds. Each C-H bond in methane, then, can be described as a sigma bond formed by overlap between a half-filled 1s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp3 hybrid orbitals in the central carbon. Step 3: Add enough electrons (dots) to the outer atoms to give each of them a total of eight electrons around them. CH 4 + Cl 2 CH 3 Cl + HCl CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl. The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. Using this formula for the oxygen atom of methanol, we have: = 6 - 4 - 2 = 0. Both the carbon and the nitrogen atom in CH3NH2aresp3-hybridized. Figuring out the formal charge on different atoms of a molecule is a straightforward process - its simply a matter of adding up valence electrons. The outer atoms are oxygen atoms, and oxygen is in group 6A, so we arent finished yet, Otherwise, create an extra bond by changing one of the nonbonding pairs into a bonding pair. Draw all of the possible constitutional isomers with the given molecular formula. a. The carbon-carbon triple bond is only 120 pm long, shorter than the double bond in ethene, and is very strong, about 837 kJ/mol. The pi bond is formed by side-by-side overlap of the unhybridized 2pz orbitals on the carbon and the oxygen. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. The alkane CH3(CH2)388CH3, in which 390 carbon atoms are bonded in a continuous chain, has been synthesized as an example of a so-called superlong alkane. The alkenes have the general formula \({C_n}{H_{2n}}\) . Next, well look at three molecules side-by-side. WebDraw the line structure of Butane. Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. How does the use of hydrocarbons affect global warming and climate change? Draw in the missing hydrogens for the following compounds. It has the correct number of electrons (32), and every atom satisfies the octet rule. They are used simply as a book-keeping methodfor predicting the most stable Lewis structure for a compound. Achieve, Sapling Learning (High School) > Drawing molecules in Assessments: Organic Chemistry. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. Do this for the structures below. Beginning with five-carbon chains, the names of unbranched alkanes consist of a Latin or Greek stem corresponding to the number of carbons in the chain followed by the suffix -ane.
Copper, for example, can be found in both its neutral state (Cu0, which is the metal), or in its Cu+2 state, as a component of an ionic compound like copper carbonate (CuCO3), the green substance called 'patina' that forms on the surface of copper objects. Using Lewis structures, we can represent this as follows: Two fluorine atoms can form a molecule of F2 in the same fashion. So which structure is best?? As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. For the purpose of calculating formal charges, however, bond dipoles dont matter - we always consider the two electrons in a bond to be shared equally, even if that is not an accurate reflection of chemical reality. -n - OH CH 2 OH Biochemistry Basics 7 00 / V. The pattern for hydrogens is easy: hydrogen atoms have only one bond, and no formal charge. The carbon-nitrogen double bond is composed of asigmabond formed from twosp2orbitals, and apibond formed from the side-by-side overlap of two unhybridized2porbitals. Methyl and ethyl are examples of alkyl groups. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. An alkyl group is derived from an alkane by deleting one of its hydrogens, thereby leaving a potential point of attachment. There is no simple arithmetic relationship between the number of carbon atoms in a formula and the number of isomers. Nomenclature in organic chemistry is of two types: common and systematic. A formal charge of -1 is located on the oxygen atom. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). Step 2: Connect the atoms to each other with single bonds to form a skeleton structure.Be sure that you follow rule 1 in the previous section. (select load sp3 and load H 1s to see orbitals). 4. Draw in the missing hydrogens for the following compounds. Another possibility is a carbon with three bonds and a single, unpaired (free radical) electron: in this case, the carbon has a formal charge of zero. Later on in this chapter and throughout this book we will see examples of organic ions called carbocations and carbanions, in which a carbon atom bears a positive or negative formal charge, respectively. In an sp-hybridized carbon, the 2s orbital combines with the 2px orbital to form two sp hybrid orbitals that are oriented at an angle of 180 with respect to each other (eg. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures. Chemists often draw square brackets around the structure of a polyatomic ion and write the charge outside the brackets, like this: Note: we could have put the double bond in two other locations. When you reach 8 electrons, youre done. { "9.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Assume that all atoms have a complete valence shell of electrons. WebThe structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. James Armstrong, City College of San Francisco, Torrey Glenn, City College of San Francisco. In this molecule, the carbon is sp2-hybridized, and we will assume that the oxygen atom is also sp2hybridized. NH, OH OH b. a. WebWhen drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect. Draw in the missing carbon and hydrogen atoms on the molecule. When there are two or more identical substituents, replicating prefixes (di-, tri-, tetra-, etc.) If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1. For methoxide, the anionic form of methanol, the calculation for the oxygen atom is: = 6 - 6 - 1 = -1. WebThe question is draw in the missing hydrogen party following compounds. Draw the missing carbon and hydrogen atoms on the molecule. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. We say that the boron atom is electron deficient. There is probably no upper limit to the number of carbon atoms possible in hydrocarbons. Draw in the missing carbon and hydrogen atoms on the molecule. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive. a. The compounds n-butane and isobutane are constitutional isomers and are the only ones possible for the formula C4H10. Nitrogen has two major bonding patterns, both of which fulfill the octet rule: If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. Draw the line structure of CHCHClCH. Omissions? In order of increasing number of carbon atoms, methane (CH4), ethane (C2H6), and propane (C3H8) are the first three members of the series. In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). And this problem gives us skeletal drawings and asks us to convert them to the letter drawings us A. Nonmetals can form a chemical bond by sharing two electrons. Updates? Step 1) Figure out how many electrons the molecule must have. The index of refraction is very high, and their glitter (sparkle or splendor) has made them the most precious stones. H 0 0 H. The Greek term iso means same. Note that it is also quite common for the central atom to makemore than four bonds. This means, in the case of ethane molecule, that the two methyl (CH3) groups can be pictured as two wheels on an axle, each one able to rotate with respect to the other. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). 2) Determine its molecular formula. WebDraw the missing carbon and hydrogen atoms on the molecule. Also, helium is shown in group 8A, but it only has two valence electrons. While every effort has been made to follow citation style rules, there may be some discrepancies.
Three experimentally observable characteristics of the ethene molecule need to be accounted for by a bonding model: Clearly, these characteristics are not consistent with an sp3 hybrid bonding picture for the two carbon atoms. Consider the Lewis structure of methanol, CH3OH (methanol is the so-called wood alcohol that unscrupulous bootleggers sometimes sold during the prohibition days in the 1920's, often causing the people who drank it to go blind). The reactions proceed via free-radical pathways, in which the halogen first dissociates into a two neutral radical atoms (homolytic fission). Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. WebDraw the line structure of Butane. Be sure to check with your instructor, but most willaccept either one.
With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a nonbonding pair (lone pair) of electrons. Alkanes with branched chains are named on the basis of the name of the longest chain of carbon atoms in the molecule, called the parent. Write the chemical formula for a molecule of noncyclic AMP. The terms aliphatic and aromatic are retained in modern terminology, but the compounds they describe are distinguished on the basis of structure rather than origin. Redraw the structures below, indicating the six atoms that lie in the same plane due to the carbon-carbon double bond. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. Occasionally, though, lone pairs are drawn if doing so helps to make an explanation more clear. Electron-deficient atoms are rare, but expanded octets are fairly common with elements in the 3rd row and beyond. Unlike a sigma bond, a pi bond does not have cylindrical symmetry.
Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects. Lets start with carbon, the most important element for organic chemists. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. If the central atom has fewer than 8 electrons around it, but all of the outer atoms are in group 7A, youre done. WebWhen drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect. This is simply a restatement of the Valence Shell Electron Pair Repulsion (VSEPR) theory that you learned in General Chemistry: electron pairs (in orbitals) will arrange themselves in such a way as to remain as far apart as possible, due to negative-negative electrostatic repulsion. Drawing all the all the carbons and hydrogen sze So this is sort of a nightmare of a problem. The most widely used standards for organic nomenclature evolved from suggestions made by a group of chemists assembled for that purpose in Geneva in 1892 and have been revised on a regular basis by the International Union of Pure and Applied Chemistry (IUPAC). Question: Question 1: 1) Redraw the molecule below, showing all of the carbons, hydrogens and other atoms. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. To do this on a two-dimensional page, though, we need to introduce a new drawing convention: the solid / dashed wedge system. One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). WebThis problem has been solved! If you look at the simple structures of methane, methanol, ethane, ethene, and ethyne in the figures from the previous section, you should quickly recognize that in each molecule, the carbon atom has four bonds, and a formal charge of zero. along the x axis). Alkenes are more reactive than alkanes because they have a double bond.
WebThe question is draw in the missing hydrogen party following compounds. It MATCHES! For example, two hydrogen atoms can form a bond, producing a molecule of H2. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. In BrF3, the central atom has 10 electrons around it. For representative elements, the number of valence electrons equals the group number on the periodic table. Join.
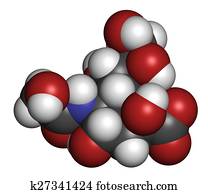 2) Determine its molecular formula. It is a poor conductor, because all electrons are localized in the chemical bonds. Write the chemical formula for a molecule of noncyclic AMP. NH OH OH b. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook.
2) Determine its molecular formula. It is a poor conductor, because all electrons are localized in the chemical bonds. Write the chemical formula for a molecule of noncyclic AMP. NH OH OH b. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Just follow the rules for drawing Lewis structures, and youll get them right! For example, the bond in O2 is a double bond. The naming of organic compounds is facilitated through the use of formal systems of nomenclature. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. The bonds on the previous sectionare called single bonds. When converted into the energy-storing molecules ADP and ATP, AMP functions as a But we can be more specific than that - we can also state for each molecular ion that a formal charge is located specifically on the oxygen atom, rather than on the carbon or any of the hydrogen atoms. b: Draw a figure showing the bonding picture for the imine below. Graph theory has been used to calculate the number of constitutionally isomeric alkanes possible for values of n in CnH2n + 2 from 1 through 400. All of these are sigma bonds. However, the phosphorus atom in the second structure violates the octet rule. Name The structures of H2, F2, and H2O would usually be drawn as follows: Only the bonding electrons are shown using lines. Remember that atoms of elements in the third row and below in the periodic table have 'expanded valence shells' with d orbitals available for bonding, and the the octet rule does not apply. Also, helium is shown in group 8A, but it only has two valence electrons. The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. If the counts do not match, add the remaining electrons to the central atom. In the crystal, every carbon atom is bonded to four other carbon atoms, and the bonds are arranged in a tetrahedral fashion. The formal charge on an atom is calculated as the number of valence electrons owned by the isolated atom minus the number of valence electrons owned by the bound atom in the molecule: (number of valence electrons owned by the isolated atom), - (number of valence electrons owned by the bound atom), - (number of non-bonding electrons on the bound atom), - ( the number of bonding electrons on the bound atom). COOH HO. Draw the line structure of CHCHClCH. When converted into the energy-storing molecules ADP and ATP, AMP functions as a 1 / 7. More than 98 percent of natural crude rubber is a hydrocarbon polymer, a chainlike molecule consisting of many units linked together.
Draw the structures of the alkene and the alcohol that react to give the following ether as the major organic product. Draw the missing carbon and hydrogen atoms on the molecule. In the hybrid orbital picture of acetylene, both carbons are sp-hybridized. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. (It will be much easier to do this if you make a model.). A hydrocarbon is any of a class of organic chemicals made up of only the elements carbon (C) and hydrogen (H). Step 5) If the central atom has 8 or more electrons around it, youre done. The carbon atom has only 6 electrons around it, so we arent finished yet. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP. Write the chemical formula for a molecule of noncyclic AMP. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. Does this match the count you got in step 1? Draw the missing hydrogen atom labels. 2) Determine its molecular formula. A dashed wedge represents a bond that is meant to be pictured pointing into, or behind, the plane of the page. Because we are concentrating in this book on organic chemistry as applied to living things, however, we will not be seeing naked protons and hydrides as such, because they are too reactive to be present in that form in aqueous solution. The molecular anion and cation have overall charges of -1 and +1, respectively. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven.