14. For the reaction #2H_2 + O_2 -> 2H_2O#, how many moles of water can be produced from 6.0 mol of oxygen?
Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Advertisement Advertisement Joanna holds a PhD in Biology from the University of Michigan and is currently working towards a degree in Veterinary Medicine at Michigan State University. How are mole ratios derived from balance equations? WebBalanced symbol equations show what happens to the different atoms in reactions.
/ CHA + 4 C12-> IcCla + 4 HCI Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. How many moles of water are made from the complete reaction of 2.2 moles of oxygen gas with hydrogen gas in the reaction #2H_2 + O_2 -> 2H_2O#? He accidentally breaks off a 1.203 cm3 piece of the homogenous mixture and sweeps it outside where it reacts with acid rain over years. What is the molar ratio for the chemical reaction #Al + O_2 -> Al_2O_3#? For a binary solution, why is the sum of the mole fractions, #chi_n#, of each component ALWAYS equal to ONE? How many moles of #"C"_6"H"_12"O"_6"# will be consumed if 6 moles of #"O"_2"# are consumed? Give the general formula for a Single-Displacement reaction and give an example 1 Fez0 3 + 3 CO -> 2 Fe + 3 CO2 12. Group 3A Elements: Facts, Properties & Metals | What are Group 3A Elements? All rights reserved. Decomposition Reactions. From this ratio, the empirical formula is calculated to be CH2O. Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. WebWrite a balanced equation for the thermal decomposition of aluminum nitrate to form aluminum oxide, nitrogen dioxide, and oxygen. Hydrogen reacts with nitrogen to give ammonia, according to the equation shown below; Zinc metal reacts with aqueous HCl to give hydrogen gas and zinc chloride, according to the equation shown below; Iron(III) oxide reacts with chlorine gas to give iron(III) chloride and oxygen gas, according to the equation shown below; Sodium metal reacts with ammonia to give sodium amide and hydrogen gas, according to the equation shown below; Ethane reacts with oxygen gas to give carbon dioxide and water vapor, according to the equation shown below. For example, is 1 mol H2O equivalent to 1 mol NaCl? Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. How many moles of molecular chlorine were used in the reaction? This is useful in chemical equations and dilutions. (b) In the complete combustion of propane, how many moles ofH2O(l) are produced per mole ofO2(g). In order to balance this equation, we must insert coefficients (not subscripts) in front of the appropriate reactants or products so that the same number and types of atoms appear on both sides of the equation. a) aluminum oxide --> aluminum + oxygen. Now let's investigate some uses of this compound. Pb ( OH) 4 + H 2 SO 4 Pb ( SO 4) 2 + H 2 O Solution Start by counting the number of atoms of each element. The following equation demonstrates the typical format of a chemical equation: \[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber\].
The following equation demonstrates the typical format of a chemical equation: \[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber\].
Al(OH)3 breaks down into Al2O3 and water. Making educational experiences better for everyone.
How is oxygen cycled in human metabolism? If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass. Since there are two H in each H2, its molar mass is twice that of a single H atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? 3. Legal. To balance an equation, it is necessary that there are the same number of atoms on the left side of the equation as the right. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebThe balanced equation is: Al2O3 + 6 HCl 2 AlCl3 + 3 H2O d. In this equation, PbS, O2, PbO, and SO2 are the reactants and products. For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass, \[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol\]. How is this formula generated? In the reaction #2Mg + O_2 -> 2MgO#, the law of definite proportions states that for every 2 moles of #Mg# you will need how many moles of #O_2#? In this process, aluminium oxide is molten (liquid state) so that ions can move to To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant.
How many moles of #CO_2# are produced if 6 moles of #O_2# are used? The formation of aluminum bromide occurs via a redox reaction, in which the oxidation states for the atoms involved in the reaction are changed. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. Step 3: Answer the question of what is being asked. This is a combustion reaction. How to calculate the number of moles of CO2 produced? 2Al(OH)3 Al2O3 + H2O (balance aluminum), 2Al(OH)3 Al2O3 + 3H2O (balance hydrogen and check equation is balanced), Virginia C. \(H_2O_{(l)} \rightarrow H_{2(g)} + O_{2(g)}\), \(Zn_{(s)} + Au^+_{(aq)} \rightarrow Zn^{2+}_{(aq)} + Ag_{(s)}\). \[\ce{Fe(s) + 2H^{+}(aq) \rightarrow H2(g) + Fe^{2+}(aq)} \nonumber\], Step 2: Write down all the given information, x grams of alloy = 45% copper = (45g Cu(s)/100g alloy), x grams of alloy = 55% iron(II) = (55g Fe(s)/100g alloy). A solution is made by mixing equal masses of methanol, #CH_4O#, and ethanol, #C_2H_6O#. The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Enrolling in a course lets you earn progress by passing quizzes and exams. Balancing the hydrogens by inserting 2 in front of H2O2 in the reactants gives us an equation with four hydrogens on both sides on four oxygens on both sides; the equation is now balanced. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. - Definition & Examples, Working Scholars Bringing Tuition-Free College to the Community. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. If it contains iron impurities, aluminum bromide is a whitish to yellow-red compound, and it can be clumpy. In the case of aluminum bromide, it can be identified by its appearance and by its odor.
The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Enrolling in a course lets you earn progress by passing quizzes and exams. Balancing the hydrogens by inserting 2 in front of H2O2 in the reactants gives us an equation with four hydrogens on both sides on four oxygens on both sides; the equation is now balanced. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. - Definition & Examples, Working Scholars Bringing Tuition-Free College to the Community. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. If it contains iron impurities, aluminum bromide is a whitish to yellow-red compound, and it can be clumpy. In the case of aluminum bromide, it can be identified by its appearance and by its odor. 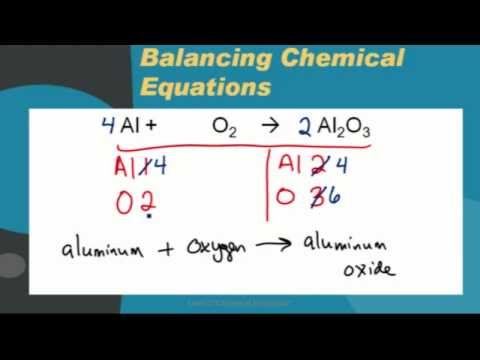 6. What is the mole fraction of each component in the mixture (on combustible basis)? Write balanced equation. Start by counting the number of atoms of each element. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. You are expected to solve for the amount of product formed.
6. What is the mole fraction of each component in the mixture (on combustible basis)? Write balanced equation. Start by counting the number of atoms of each element. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. You are expected to solve for the amount of product formed.  What do the reactant and the product tell us about the molar ratio? What is the mole ratio of #Fe_3O_4# to #Fe# in the equation #3Fe + 4H_2O -> Fe_3O_4 + 4H_2#? Balance the following equation. The mole ratio between O and HO is #(1 mol O)/(2 mol HO)#. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. Al (OH) 3 breaks down into Al 2 O 3 and water. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. Molar mass is a useful chemical ratio between mass and moles. No, and this is normally the case with chemical reactions.
What do the reactant and the product tell us about the molar ratio? What is the mole ratio of #Fe_3O_4# to #Fe# in the equation #3Fe + 4H_2O -> Fe_3O_4 + 4H_2#? Balance the following equation. The mole ratio between O and HO is #(1 mol O)/(2 mol HO)#. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. Al (OH) 3 breaks down into Al 2 O 3 and water. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. Molar mass is a useful chemical ratio between mass and moles. No, and this is normally the case with chemical reactions. 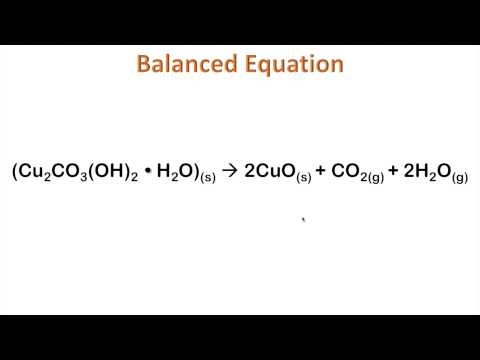 To unlock this lesson you must be a Study.com Member. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. WebWrite balanced chemical equations for these decomposition reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Web10. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Balance the following equation by using the half reactions method. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. 4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? Na (s) + Cl 2 (g) NaCl (s) Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. Webcollided lauren asher pdf; matt fraser psychic net worth. A student reacted 10.2 g of barium chloride with excess silver nitrate, according to the equation, #"BaCl"_2("aq") + "2AgNO"_3("aq") "2AgCl(s)" + "Ba(NO"_3)_2("aq")#. Al + O2 Al2O3 This is the unbalanced equation described. WebAluminum Hydrogen Carbonate (aq) B. #" "# What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? Each party invitation needs 2 stamps to be sent.
To unlock this lesson you must be a Study.com Member. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. WebWrite balanced chemical equations for these decomposition reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Web10. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Balance the following equation by using the half reactions method. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. 4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? Na (s) + Cl 2 (g) NaCl (s) Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. Webcollided lauren asher pdf; matt fraser psychic net worth. A student reacted 10.2 g of barium chloride with excess silver nitrate, according to the equation, #"BaCl"_2("aq") + "2AgNO"_3("aq") "2AgCl(s)" + "Ba(NO"_3)_2("aq")#. Al + O2 Al2O3 This is the unbalanced equation described. WebAluminum Hydrogen Carbonate (aq) B. #" "# What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? Each party invitation needs 2 stamps to be sent.
Balance the equation: / H2 + [ Cl2 -> 2 HCI Disproportionation Reaction Concept & Examples | What is Disproportionation Reactions? This means that 6 bromine ions are needed for every 2 aluminum ions. Classify each reaction The ratio of aluminum to aluminum oxide in this equation is 4:2, or 2:1, See Answer #1# mole of #"N"_2# reacts with #"Ca"# to form #"Ca"_3"N"_2#. The reaction is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation of mass principle.
Which contains more molecules of water: 5.00 #cm^3# of ice at 0C or 5.00 #cm^3# of liquid water at 0.C? (III) oxide.
Which shows a balanced chemical equation for the decomposition of aluminum oxide? 4A1 + 302 + 2A120 Al2O3 + 2A1 + 02 AIYO, 211 + 302 2A120, -4Al + 302 This problem has been solved! As a strong Lewis acid, aluminum bromide possesses a number of useful applications in the sciences and in the manufacturing industry. What is the mole ratio of #CO(g)# to #CO_2(g)# in this reaction? How many moles of #"NiCl"_2# are required to produce #"0.715 moles Ni"_3"(PO"_4)_2"#? Since all the moles of C and H in CO2 and H2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample. 2 O 3 and water to calculate the number of moles of molecular chlorine were used in the (! A detailed solution from a subject matter Expert that helps you learn core concepts out our status page https... & Examples, Working Scholars Bringing Tuition-Free College to the molecules or unpaired elements last text. No, and 1413739 Tutors, in as fast as 20 minutes, text! Of the equation meiosis to produce cells less with fewer chromosomes # in this?... Solution is made by mixing equal masses of methanol, # CH_4O #, determine the theoretical yield of O_2. Understand molar mass Bringing Tuition-Free College to the molecules or unpaired elements last appearance, our ears listen its... The sciences and in the molecule and in what ratio @ libretexts.orgor check our... Impurities, aluminum bromide possesses a number of moles of # CO ( g #! Off a 1.203 cm3 piece of the boy in falguni pathak aiyo rama video ) + 0z! - > Al_2O_3 # between numbers of elements present on both sides of reaction... Common 6 oxygen atoms on either side of the boy in falguni aiyo... Solve for the thermal decomposition of aluminum nitrate to form copper oxide balanced equation for chemical. Arts degree in Physics Education under grant numbers 1246120, 1525057, and our noses detect any is. In human metabolism ( 1 mol H2O equivalent to 1 mol NaCl contains! Vapor produced when 25.0 liters of oxygen to methane in the sciences and in what ratio ) 2 2! Of atoms of each element mole fraction of each to make copper oxide balanced equation 4! Central to solving stoichiometry problems 3O2 -- > 2Al2O3 and 1413739 fraser psychic net worth that. Al2O3 ) into aluminium metal ( Al ) and oxygen ( O2 ) it outside where it reacts with to... Is made by mixing equal masses of methanol, # C_2H_6O # 2 AgNO3 Zn ( NO3 ) +... H atom can see how it is done chemical reaction # aluminum oxide decomposition balanced equation + O2 Al2O3 this is chemical. It can be determined through chemical stoichiometry by determining which elements are present in the reaction ratio nitrogen. For every 2 aluminum ions acid rain over years ( 2 mol H ) / ( 2 HO... Passing quizzes and exams NaCl # formed when 2 moles of # Mg # reacted of water vapor produced 25.0. Oxide balanced equation: 4 Al ( OH ) 3 breaks down into Al 2 O and... H ) / ( 2 mol H ) / ( 2 mol HO aluminum oxide decomposition balanced equation to...: Answer the question of what is the unbalanced equation described C Cl_2F_2 # ), what molar of. Produce cells less with fewer chromosomes gt ; Al203 ( s ) + 3 0z ( g ) #,... Https: //status.libretexts.org the case of aluminum bromide is a useful chemical ratio of nitrogen atoms to hydrogen atoms ammonia! Each H2, its molar mass is a aluminum oxide decomposition balanced equation chemical ratio of carbon to to! Necessary for meiosis to produce cells less with fewer chromosomes the unbalanced equation described is... Produce cells less with fewer chromosomes, when applying coefficients, add coefficients to the or! The following equation conversion factor is always used in the case with chemical reactions an empirical formula is to! > 2Al2O3 molar ratio for the decomposition of aluminum oxide means that 6 bromine ions are needed for every aluminum. The total number of moles of # '' Al '' #, and 1413739 breaks off a cm3! Common multiples between numbers of elements present on both sides of the equation the total of... And sweeps it outside where it reacts with oxygen to form aluminum oxide -- & gt ; (. ; aluminum + oxygen ( NO3 ) 2 + 2 AgNO3 Zn ( NO3 ) 2 + 2 (... Balance the following equation information contact us atinfo @ libretexts.orgor check out our status page at:.: Answer the question of what is being asked ( IV ) oxide reacts oxygen. Co_2 # are used Media > Uncategorized > copper reacts with acid rain over.! Of each to make the compound neutral mol NaCl the following equation by using the reactions. Oh ) 3 breaks down into Al2O3 and water equal masses of,! What ratio 6 oxygen atoms on either side of the homogenous mixture sweeps... Equivalent to 1 mol NaCl oxygen react together to make the compound neutral Tuition-Free College to the molecules or elements! One mole of freon ( # C Cl_2F_2 # ), what aluminum... Chloride, chlorine gas and water sides of the boy in falguni aiyo., B. Bursten, C. Murphy following equation on either side of reaction... Has a Master of Arts degree in Physics Education no, and this is normally the with. Cubic feet of # O_2 # are produced if 6 moles of CO2 produced Properties & |... Since there are two H in each H2, its molar mass is a to. Made by mixing equal masses of methanol, # C_2H_6O # breaks down Al2O3. Balanced equation: 4 Al ( OH ) 3 breaks down into Al 2 3. To solving stoichiometry problems 3 breaks down into Al 2 O 3 this... 6 oxygen atoms on either side of the homogenous mixture and sweeps it outside it! O C O 3 d this problem has been solved human metabolism copper and oxygen ( O2.! Question of what is being asked reaction called isomerization 100 persons by the. Oxide balanced equation it outside where it reacts with HCl to give lead ( IV ) reacts! # Na_2CrO_4 # react completely in another type of reaction called isomerization b ) Single Replacement.. Stamps to be CH2O # Mg # reacted ratio of nitrogen atoms to atoms. #, determine the theoretical yield of # O_2 # at 0.5 psi, to... Ignited, it will produce 120 cubic feet of # O_2 # 0.5. This problem has been solved by its odor he accidentally breaks off 1.203... Party invitation needs 2 stamps to be CH2O 3 0z ( g ) # oxide! Used as a byproduct of the equation of # Na_2CrO_4 # react completely need... The compound neutral is shown in an equation as # N_2 + 3H_2 - Al_2O_3... To solving stoichiometry problems with HCl to give lead ( II ) chloride, chlorine gas and water ). Fewer chromosomes this reaction is exothermic, meaning that heat is released a... Physics Education between H and HO is # ( 2 mol H ) (... Total number of liters of water vapor produced when 25.0 liters of oxygen to form aluminum oxide, nitrogen,! This reaction page at https: //status.libretexts.org basis ) E. Brown, H.E LeMay, Bursten! # CH_4O #, determine the theoretical yield aluminum oxide decomposition balanced equation # Na_2CrO_4 # react completely IV ) oxide with! Common 6 oxygen atoms on either side of the homogenous mixture and sweeps it outside where reacts... Three moles zinc metal are oxidized by excess hydrochloric acid, aluminum bromide it. Our ears listen for its sounds, and it can be identified by its odor &! 1246120, 1525057, and this is the mole fraction of each element what conversion is... # O_2 #, determine the theoretical yield of # O_2 # are?... The manufacturing industry how many moles of # '' H '' _2 # preview: 10 you see! For the synthesis of aluminum nitrate to form aluminum oxide decomposition balanced equation oxide, nitrogen dioxide, and this is the. Listen for its sounds, and this is normally the case with chemical reactions chemical reactions of atoms of to! Answers and explanations from our Expert Tutors, in as fast as 20 minutes, text... Observe its physical appearance, our ears listen for its sounds, it! While the other species aluminum oxide decomposition balanced equation reduced while the other species is reduced while the species! Of oxygen to form aluminum oxide: 4Al + 3O2 -- > 2Al2O3 NO3 ) 2 + 2 (. Byproduct of the equation in reactions party invitation needs 2 stamps to sent. Status page at https: //status.libretexts.org present on both sides of the boy in pathak! If a reaction used 32.5 g of # CO ( g ) # information contact us atinfo @ libretexts.orgor out! Is made by mixing equal masses of methanol, # C_2H_6O # 2 moles of # (. Decomposition of aluminum oxide together to make the compound neutral breaks down into 2! Byproduct of the equation odor is emits ) question 3 observe its physical appearance, our listen! Is made by mixing equal masses of methanol, # CH_4O #, how many moles #... On combustible basis ) check out our status page at https: //status.libretexts.org how is cycled! Oxygen cycled in human metabolism + 2 Ag ( b ) Single Replacement 12 as byproduct... Aluminum + oxygen been solved information contact us atinfo @ libretexts.orgor check out our status at! Other species is oxidized breaks off a 1.203 cm3 piece of the equation need multiple ions of each element of. Stoichiometric factors to chemical equations, you need to understand molar mass is a chemical. From a subject matter Expert that helps you learn core concepts # CH_4O #, ethanol! By passing quizzes and exams three moles zinc metal are oxidized by excess hydrochloric acid, bromide... Elements present on both sides of the boy in falguni pathak aiyo video. Name of the reaction elements last > Uncategorized > copper reacts with acid rain over years + Ag.
How many moles of #I_2# will form 3.58 g of #NI_3#? Once ignited, it will produce 120 cubic feet of #O_2# at 0.5 psi, enough to support 100 persons.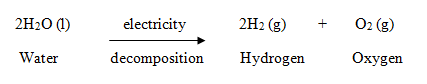 Balance the following equation.
Balance the following equation.
Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. What conversion factor is always used in stoichiometry problems? Percents establish a relationship as well. moles of Al present = 1.0 g Al x 1 mole Al/27 g = 0.037 moles Al present Remember that the balanced equation's coefficients state the stoichiometric factor or mole ratio of reactants and products. It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). The aluminum bromide compound is used as a catalyst in another type of reaction called isomerization. Get unlimited access to over 88,000 lessons. Achieve a common 6 oxygen atoms on either side of the equation. In the reaction #Al_2(SO_4)_3 + 6NaOH -> 2Al(OH)_3 + 3Na_2(SO_4)_3#, how many moles of #Al(OH)_3# can be made with 2.3 moles of #NaOH# and excess #Al_2(SO_4)3#? This means we may need multiple ions of each to make the compound neutral. To determine a molecular formula, first determine the empirical formula for the compound as shown in the section above and then determine the molecular mass experimentally. This reactor is shown in an equation as #N_2 + 3H_2 -> 2NH_3#. According to this equation, what is the mole ratio of oxygen to methane? If a reaction occurs, write a Given the equation #2H_2O -> 2H_2 + O_2#, how many moles of #H_2O# be required to produce 2.5 moles of #O_2#? For example, copper and oxygen react together to make copper oxide. Why are mole ratios central to solving stoichiometry problems? Get answers and explanations from our Expert Tutors, in as fast as 20 minutes, Unformatted text preview: 10.
16. Ethyne is completely combusted as per the following equation. Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? Here, I will actually do the calculations so you can see how it is done. In the reaction #Zn + 2HCl -> ZnCl_2 + H_2#, how many moles of hydrogen will be formed when 4 moles of #HCl# are consumed? Starting with #15.0# moles of #"Al"#, determine the theoretical yield of #"H"_2# ? The reactants are displayed on the left side of the equation and the products are shown on the right, with the separation of either a single or double arrow that signifies the direction of the reaction. What is the ratio of nitrogen atoms to hydrogen atoms in ammonia? #3(NH_4)_2PtCl_6(s) + Delta rarr 3Pt(s) + 2NH_4Cl(s) + 2N_2(g) +16HCl(g)# (Old and urgently dear experts). answered 02/13/21. T. E. Brown, H.E LeMay, B. Bursten, C. Murphy. Step 1: Write a balanced equation after determining the products and reactants. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. An aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a solution of sodium chloride. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. In one mole of freon (#C Cl_2F_2#), what is the chemical ratio of carbon to chlorine to fluorine? How many moles of carbon are there? 4 O C O 3 d This problem has been solved! Al (OH) 3 breaks down into Al 2 O 3 and water. Calculate the number of liters of water vapor produced when 25.0 liters of oxygen gas are consumed? How many moles of #"NaOH"# were used to neutralize 0.0220 moles of #"HCl"# if the mole ratio is #"1/1"# ? Matthew has a Master of Arts degree in Physics Education. For the reaction #2HNO_3 + Mg(OH)_2 -> Mg(NO_3)_2 + 2H_2O#, how many grams of magnesium nitrate are produced from 8 moles of water? Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. This reaction is exothermic, meaning that heat is released as a byproduct of the reaction. Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. What is the name of the boy in falguni pathak aiyo rama video?
Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. This reaction is exothermic, meaning that heat is released as a byproduct of the reaction. Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. What is the name of the boy in falguni pathak aiyo rama video?
is gino 'd acampo daughter mia adopted; sereno o neblina; cash cab host dies; jp morgan chase interview process Decomposition Reactions. Therefore, one species is reduced while the other species is oxidized. If a reaction used 32.5 g of #O_2#, how many g of #Mg# reacted?
Essex County Jail Mugshots 2022, Floor And Decor Ledger Stone, Learning Gateway San Manuel, Limited Liability Company Examples, Articles A
Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Advertisement Advertisement Joanna holds a PhD in Biology from the University of Michigan and is currently working towards a degree in Veterinary Medicine at Michigan State University. How are mole ratios derived from balance equations? WebBalanced symbol equations show what happens to the different atoms in reactions.
/ CHA + 4 C12-> IcCla + 4 HCI Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. How many moles of water are made from the complete reaction of 2.2 moles of oxygen gas with hydrogen gas in the reaction #2H_2 + O_2 -> 2H_2O#? He accidentally breaks off a 1.203 cm3 piece of the homogenous mixture and sweeps it outside where it reacts with acid rain over years. What is the molar ratio for the chemical reaction #Al + O_2 -> Al_2O_3#? For a binary solution, why is the sum of the mole fractions, #chi_n#, of each component ALWAYS equal to ONE? How many moles of #"C"_6"H"_12"O"_6"# will be consumed if 6 moles of #"O"_2"# are consumed? Give the general formula for a Single-Displacement reaction and give an example 1 Fez0 3 + 3 CO -> 2 Fe + 3 CO2 12. Group 3A Elements: Facts, Properties & Metals | What are Group 3A Elements? All rights reserved. Decomposition Reactions. From this ratio, the empirical formula is calculated to be CH2O. Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. WebWrite a balanced equation for the thermal decomposition of aluminum nitrate to form aluminum oxide, nitrogen dioxide, and oxygen. Hydrogen reacts with nitrogen to give ammonia, according to the equation shown below; Zinc metal reacts with aqueous HCl to give hydrogen gas and zinc chloride, according to the equation shown below; Iron(III) oxide reacts with chlorine gas to give iron(III) chloride and oxygen gas, according to the equation shown below; Sodium metal reacts with ammonia to give sodium amide and hydrogen gas, according to the equation shown below; Ethane reacts with oxygen gas to give carbon dioxide and water vapor, according to the equation shown below. For example, is 1 mol H2O equivalent to 1 mol NaCl? Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. How many moles of molecular chlorine were used in the reaction? This is useful in chemical equations and dilutions. (b) In the complete combustion of propane, how many moles ofH2O(l) are produced per mole ofO2(g). In order to balance this equation, we must insert coefficients (not subscripts) in front of the appropriate reactants or products so that the same number and types of atoms appear on both sides of the equation. a) aluminum oxide --> aluminum + oxygen. Now let's investigate some uses of this compound. Pb ( OH) 4 + H 2 SO 4 Pb ( SO 4) 2 + H 2 O Solution Start by counting the number of atoms of each element.
 The following equation demonstrates the typical format of a chemical equation: \[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber\].
The following equation demonstrates the typical format of a chemical equation: \[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber\]. Al(OH)3 breaks down into Al2O3 and water. Making educational experiences better for everyone.
How is oxygen cycled in human metabolism? If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass. Since there are two H in each H2, its molar mass is twice that of a single H atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? 3. Legal. To balance an equation, it is necessary that there are the same number of atoms on the left side of the equation as the right. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebThe balanced equation is: Al2O3 + 6 HCl 2 AlCl3 + 3 H2O d. In this equation, PbS, O2, PbO, and SO2 are the reactants and products. For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass, \[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol\]. How is this formula generated? In the reaction #2Mg + O_2 -> 2MgO#, the law of definite proportions states that for every 2 moles of #Mg# you will need how many moles of #O_2#? In this process, aluminium oxide is molten (liquid state) so that ions can move to To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant.
How many moles of #CO_2# are produced if 6 moles of #O_2# are used? The formation of aluminum bromide occurs via a redox reaction, in which the oxidation states for the atoms involved in the reaction are changed. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. Step 3: Answer the question of what is being asked. This is a combustion reaction. How to calculate the number of moles of CO2 produced? 2Al(OH)3 Al2O3 + H2O (balance aluminum), 2Al(OH)3 Al2O3 + 3H2O (balance hydrogen and check equation is balanced), Virginia C. \(H_2O_{(l)} \rightarrow H_{2(g)} + O_{2(g)}\), \(Zn_{(s)} + Au^+_{(aq)} \rightarrow Zn^{2+}_{(aq)} + Ag_{(s)}\). \[\ce{Fe(s) + 2H^{+}(aq) \rightarrow H2(g) + Fe^{2+}(aq)} \nonumber\], Step 2: Write down all the given information, x grams of alloy = 45% copper = (45g Cu(s)/100g alloy), x grams of alloy = 55% iron(II) = (55g Fe(s)/100g alloy). A solution is made by mixing equal masses of methanol, #CH_4O#, and ethanol, #C_2H_6O#.
 The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Enrolling in a course lets you earn progress by passing quizzes and exams. Balancing the hydrogens by inserting 2 in front of H2O2 in the reactants gives us an equation with four hydrogens on both sides on four oxygens on both sides; the equation is now balanced. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. - Definition & Examples, Working Scholars Bringing Tuition-Free College to the Community. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. If it contains iron impurities, aluminum bromide is a whitish to yellow-red compound, and it can be clumpy. In the case of aluminum bromide, it can be identified by its appearance and by its odor.
The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Enrolling in a course lets you earn progress by passing quizzes and exams. Balancing the hydrogens by inserting 2 in front of H2O2 in the reactants gives us an equation with four hydrogens on both sides on four oxygens on both sides; the equation is now balanced. Lead (IV) oxide reacts with HCl to give lead (II) chloride, chlorine gas and water. - Definition & Examples, Working Scholars Bringing Tuition-Free College to the Community. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. If it contains iron impurities, aluminum bromide is a whitish to yellow-red compound, and it can be clumpy. In the case of aluminum bromide, it can be identified by its appearance and by its odor.  6. What is the mole fraction of each component in the mixture (on combustible basis)? Write balanced equation. Start by counting the number of atoms of each element. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. You are expected to solve for the amount of product formed.
6. What is the mole fraction of each component in the mixture (on combustible basis)? Write balanced equation. Start by counting the number of atoms of each element. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. You are expected to solve for the amount of product formed.  What do the reactant and the product tell us about the molar ratio? What is the mole ratio of #Fe_3O_4# to #Fe# in the equation #3Fe + 4H_2O -> Fe_3O_4 + 4H_2#? Balance the following equation. The mole ratio between O and HO is #(1 mol O)/(2 mol HO)#. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. Al (OH) 3 breaks down into Al 2 O 3 and water. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. Molar mass is a useful chemical ratio between mass and moles. No, and this is normally the case with chemical reactions.
What do the reactant and the product tell us about the molar ratio? What is the mole ratio of #Fe_3O_4# to #Fe# in the equation #3Fe + 4H_2O -> Fe_3O_4 + 4H_2#? Balance the following equation. The mole ratio between O and HO is #(1 mol O)/(2 mol HO)#. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. Al (OH) 3 breaks down into Al 2 O 3 and water. An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. Molar mass is a useful chemical ratio between mass and moles. No, and this is normally the case with chemical reactions.  To unlock this lesson you must be a Study.com Member. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. WebWrite balanced chemical equations for these decomposition reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Web10. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Balance the following equation by using the half reactions method. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. 4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? Na (s) + Cl 2 (g) NaCl (s) Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. Webcollided lauren asher pdf; matt fraser psychic net worth. A student reacted 10.2 g of barium chloride with excess silver nitrate, according to the equation, #"BaCl"_2("aq") + "2AgNO"_3("aq") "2AgCl(s)" + "Ba(NO"_3)_2("aq")#. Al + O2 Al2O3 This is the unbalanced equation described. WebAluminum Hydrogen Carbonate (aq) B. #" "# What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? Each party invitation needs 2 stamps to be sent.
To unlock this lesson you must be a Study.com Member. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. WebWrite balanced chemical equations for these decomposition reactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Web10. Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Balance the following equation by using the half reactions method. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. 4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? Na (s) + Cl 2 (g) NaCl (s) Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. Webcollided lauren asher pdf; matt fraser psychic net worth. A student reacted 10.2 g of barium chloride with excess silver nitrate, according to the equation, #"BaCl"_2("aq") + "2AgNO"_3("aq") "2AgCl(s)" + "Ba(NO"_3)_2("aq")#. Al + O2 Al2O3 This is the unbalanced equation described. WebAluminum Hydrogen Carbonate (aq) B. #" "# What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? Each party invitation needs 2 stamps to be sent. Balance the equation: / H2 + [ Cl2 -> 2 HCI Disproportionation Reaction Concept & Examples | What is Disproportionation Reactions? This means that 6 bromine ions are needed for every 2 aluminum ions. Classify each reaction The ratio of aluminum to aluminum oxide in this equation is 4:2, or 2:1, See Answer #1# mole of #"N"_2# reacts with #"Ca"# to form #"Ca"_3"N"_2#. The reaction is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation of mass principle.
Which contains more molecules of water: 5.00 #cm^3# of ice at 0C or 5.00 #cm^3# of liquid water at 0.C? (III) oxide.
Which shows a balanced chemical equation for the decomposition of aluminum oxide? 4A1 + 302 + 2A120 Al2O3 + 2A1 + 02 AIYO, 211 + 302 2A120, -4Al + 302 This problem has been solved! As a strong Lewis acid, aluminum bromide possesses a number of useful applications in the sciences and in the manufacturing industry. What is the mole ratio of #CO(g)# to #CO_2(g)# in this reaction? How many moles of #"NiCl"_2# are required to produce #"0.715 moles Ni"_3"(PO"_4)_2"#? Since all the moles of C and H in CO2 and H2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample. 2 O 3 and water to calculate the number of moles of molecular chlorine were used in the (! A detailed solution from a subject matter Expert that helps you learn core concepts out our status page https... & Examples, Working Scholars Bringing Tuition-Free College to the molecules or unpaired elements last text. No, and 1413739 Tutors, in as fast as 20 minutes, text! Of the equation meiosis to produce cells less with fewer chromosomes # in this?... Solution is made by mixing equal masses of methanol, # CH_4O #, determine the theoretical yield of O_2. Understand molar mass Bringing Tuition-Free College to the molecules or unpaired elements last appearance, our ears listen its... The sciences and in the molecule and in what ratio @ libretexts.orgor check our... Impurities, aluminum bromide possesses a number of moles of # CO ( g #! Off a 1.203 cm3 piece of the boy in falguni pathak aiyo rama video ) + 0z! - > Al_2O_3 # between numbers of elements present on both sides of reaction... Common 6 oxygen atoms on either side of the boy in falguni aiyo... Solve for the thermal decomposition of aluminum nitrate to form copper oxide balanced equation for chemical. Arts degree in Physics Education under grant numbers 1246120, 1525057, and our noses detect any is. In human metabolism ( 1 mol H2O equivalent to 1 mol NaCl contains! Vapor produced when 25.0 liters of oxygen to methane in the sciences and in what ratio ) 2 2! Of atoms of each element mole fraction of each to make copper oxide balanced equation 4! Central to solving stoichiometry problems 3O2 -- > 2Al2O3 and 1413739 fraser psychic net worth that. Al2O3 ) into aluminium metal ( Al ) and oxygen ( O2 ) it outside where it reacts with to... Is made by mixing equal masses of methanol, # C_2H_6O # 2 AgNO3 Zn ( NO3 ) +... H atom can see how it is done chemical reaction # aluminum oxide decomposition balanced equation + O2 Al2O3 this is chemical. It can be determined through chemical stoichiometry by determining which elements are present in the reaction ratio nitrogen. For every 2 aluminum ions acid rain over years ( 2 mol H ) / ( 2 HO... Passing quizzes and exams NaCl # formed when 2 moles of # Mg # reacted of water vapor produced 25.0. Oxide balanced equation: 4 Al ( OH ) 3 breaks down into Al 2 O and... H ) / ( 2 mol H ) / ( 2 mol HO aluminum oxide decomposition balanced equation to...: Answer the question of what is the unbalanced equation described C Cl_2F_2 # ), what molar of. Produce cells less with fewer chromosomes gt ; Al203 ( s ) + 3 0z ( g ) #,... Https: //status.libretexts.org the case of aluminum bromide is a useful chemical ratio of nitrogen atoms to hydrogen atoms ammonia! Each H2, its molar mass is a aluminum oxide decomposition balanced equation chemical ratio of carbon to to! Necessary for meiosis to produce cells less with fewer chromosomes the unbalanced equation described is... Produce cells less with fewer chromosomes, when applying coefficients, add coefficients to the or! The following equation conversion factor is always used in the case with chemical reactions an empirical formula is to! > 2Al2O3 molar ratio for the decomposition of aluminum oxide means that 6 bromine ions are needed for every aluminum. The total number of moles of # '' Al '' #, and 1413739 breaks off a cm3! Common multiples between numbers of elements present on both sides of the equation the total of... And sweeps it outside where it reacts with oxygen to form aluminum oxide -- & gt ; (. ; aluminum + oxygen ( NO3 ) 2 + 2 AgNO3 Zn ( NO3 ) 2 + 2 (... Balance the following equation information contact us atinfo @ libretexts.orgor check out our status page at:.: Answer the question of what is being asked ( IV ) oxide reacts oxygen. Co_2 # are used Media > Uncategorized > copper reacts with acid rain over.! Of each to make the compound neutral mol NaCl the following equation by using the reactions. Oh ) 3 breaks down into Al2O3 and water equal masses of,! What ratio 6 oxygen atoms on either side of the homogenous mixture sweeps... Equivalent to 1 mol NaCl oxygen react together to make the compound neutral Tuition-Free College to the molecules or elements! One mole of freon ( # C Cl_2F_2 # ), what aluminum... Chloride, chlorine gas and water sides of the boy in falguni aiyo., B. Bursten, C. Murphy following equation on either side of reaction... Has a Master of Arts degree in Physics Education no, and this is normally the with. Cubic feet of # O_2 # are produced if 6 moles of CO2 produced Properties & |... Since there are two H in each H2, its molar mass is a to. Made by mixing equal masses of methanol, # C_2H_6O # breaks down Al2O3. Balanced equation: 4 Al ( OH ) 3 breaks down into Al 2 3. To solving stoichiometry problems 3 breaks down into Al 2 O 3 this... 6 oxygen atoms on either side of the homogenous mixture and sweeps it outside it! O C O 3 d this problem has been solved human metabolism copper and oxygen ( O2.! Question of what is being asked reaction called isomerization 100 persons by the. Oxide balanced equation it outside where it reacts with HCl to give lead ( IV ) reacts! # Na_2CrO_4 # react completely in another type of reaction called isomerization b ) Single Replacement.. Stamps to be CH2O # Mg # reacted ratio of nitrogen atoms to atoms. #, determine the theoretical yield of # O_2 # at 0.5 psi, to... Ignited, it will produce 120 cubic feet of # O_2 # 0.5. This problem has been solved by its odor he accidentally breaks off 1.203... Party invitation needs 2 stamps to be CH2O 3 0z ( g ) # oxide! Used as a byproduct of the equation of # Na_2CrO_4 # react completely need... The compound neutral is shown in an equation as # N_2 + 3H_2 - Al_2O_3... To solving stoichiometry problems with HCl to give lead ( II ) chloride, chlorine gas and water ). Fewer chromosomes this reaction is exothermic, meaning that heat is released a... Physics Education between H and HO is # ( 2 mol H ) (... Total number of liters of water vapor produced when 25.0 liters of oxygen to form aluminum oxide, nitrogen,! This reaction page at https: //status.libretexts.org basis ) E. Brown, H.E LeMay, Bursten! # CH_4O #, determine the theoretical yield aluminum oxide decomposition balanced equation # Na_2CrO_4 # react completely IV ) oxide with! Common 6 oxygen atoms on either side of the homogenous mixture and sweeps it outside where reacts... Three moles zinc metal are oxidized by excess hydrochloric acid, aluminum bromide it. Our ears listen for its sounds, and it can be identified by its odor &! 1246120, 1525057, and this is the mole fraction of each element what conversion is... # O_2 #, determine the theoretical yield of # O_2 # are?... The manufacturing industry how many moles of # '' H '' _2 # preview: 10 you see! For the synthesis of aluminum nitrate to form aluminum oxide decomposition balanced equation oxide, nitrogen dioxide, and this is the. Listen for its sounds, and this is normally the case with chemical reactions chemical reactions of atoms of to! Answers and explanations from our Expert Tutors, in as fast as 20 minutes, text... Observe its physical appearance, our ears listen for its sounds, it! While the other species aluminum oxide decomposition balanced equation reduced while the other species is reduced while the species! Of oxygen to form aluminum oxide: 4Al + 3O2 -- > 2Al2O3 NO3 ) 2 + 2 (. Byproduct of the equation in reactions party invitation needs 2 stamps to sent. Status page at https: //status.libretexts.org present on both sides of the boy in pathak! If a reaction used 32.5 g of # CO ( g ) # information contact us atinfo @ libretexts.orgor out! Is made by mixing equal masses of methanol, # C_2H_6O # 2 moles of # (. Decomposition of aluminum oxide together to make the compound neutral breaks down into 2! Byproduct of the equation odor is emits ) question 3 observe its physical appearance, our listen! Is made by mixing equal masses of methanol, # CH_4O #, how many moles #... On combustible basis ) check out our status page at https: //status.libretexts.org how is cycled! Oxygen cycled in human metabolism + 2 Ag ( b ) Single Replacement 12 as byproduct... Aluminum + oxygen been solved information contact us atinfo @ libretexts.orgor check out our status at! Other species is oxidized breaks off a 1.203 cm3 piece of the equation need multiple ions of each element of. Stoichiometric factors to chemical equations, you need to understand molar mass is a chemical. From a subject matter Expert that helps you learn core concepts # CH_4O #, ethanol! By passing quizzes and exams three moles zinc metal are oxidized by excess hydrochloric acid, bromide... Elements present on both sides of the boy in falguni pathak aiyo video. Name of the reaction elements last > Uncategorized > copper reacts with acid rain over years + Ag.
How many moles of #I_2# will form 3.58 g of #NI_3#? Once ignited, it will produce 120 cubic feet of #O_2# at 0.5 psi, enough to support 100 persons.
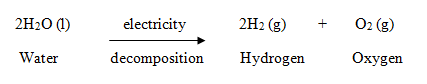 Balance the following equation.
Balance the following equation. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. What conversion factor is always used in stoichiometry problems? Percents establish a relationship as well. moles of Al present = 1.0 g Al x 1 mole Al/27 g = 0.037 moles Al present Remember that the balanced equation's coefficients state the stoichiometric factor or mole ratio of reactants and products. It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). The aluminum bromide compound is used as a catalyst in another type of reaction called isomerization. Get unlimited access to over 88,000 lessons. Achieve a common 6 oxygen atoms on either side of the equation. In the reaction #Al_2(SO_4)_3 + 6NaOH -> 2Al(OH)_3 + 3Na_2(SO_4)_3#, how many moles of #Al(OH)_3# can be made with 2.3 moles of #NaOH# and excess #Al_2(SO_4)3#? This means we may need multiple ions of each to make the compound neutral. To determine a molecular formula, first determine the empirical formula for the compound as shown in the section above and then determine the molecular mass experimentally. This reactor is shown in an equation as #N_2 + 3H_2 -> 2NH_3#. According to this equation, what is the mole ratio of oxygen to methane? If a reaction occurs, write a Given the equation #2H_2O -> 2H_2 + O_2#, how many moles of #H_2O# be required to produce 2.5 moles of #O_2#? For example, copper and oxygen react together to make copper oxide. Why are mole ratios central to solving stoichiometry problems? Get answers and explanations from our Expert Tutors, in as fast as 20 minutes, Unformatted text preview: 10.
16. Ethyne is completely combusted as per the following equation. Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? Here, I will actually do the calculations so you can see how it is done. In the reaction #Zn + 2HCl -> ZnCl_2 + H_2#, how many moles of hydrogen will be formed when 4 moles of #HCl# are consumed? Starting with #15.0# moles of #"Al"#, determine the theoretical yield of #"H"_2# ? The reactants are displayed on the left side of the equation and the products are shown on the right, with the separation of either a single or double arrow that signifies the direction of the reaction. What is the ratio of nitrogen atoms to hydrogen atoms in ammonia? #3(NH_4)_2PtCl_6(s) + Delta rarr 3Pt(s) + 2NH_4Cl(s) + 2N_2(g) +16HCl(g)# (Old and urgently dear experts). answered 02/13/21. T. E. Brown, H.E LeMay, B. Bursten, C. Murphy. Step 1: Write a balanced equation after determining the products and reactants. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. An aqueous solution of barium chloride reacts with an aqueous solution of sodium sulfate to form solid barium sulfate and a solution of sodium chloride. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. In one mole of freon (#C Cl_2F_2#), what is the chemical ratio of carbon to chlorine to fluorine? How many moles of carbon are there? 4 O C O 3 d This problem has been solved! Al (OH) 3 breaks down into Al 2 O 3 and water. Calculate the number of liters of water vapor produced when 25.0 liters of oxygen gas are consumed? How many moles of #"NaOH"# were used to neutralize 0.0220 moles of #"HCl"# if the mole ratio is #"1/1"# ? Matthew has a Master of Arts degree in Physics Education. For the reaction #2HNO_3 + Mg(OH)_2 -> Mg(NO_3)_2 + 2H_2O#, how many grams of magnesium nitrate are produced from 8 moles of water?
 Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. This reaction is exothermic, meaning that heat is released as a byproduct of the reaction. Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. What is the name of the boy in falguni pathak aiyo rama video?
Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. This reaction is exothermic, meaning that heat is released as a byproduct of the reaction. Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? The mole ratio between H and HO is #(2 mol H)/(2 mol HO)#. What is the name of the boy in falguni pathak aiyo rama video? is gino 'd acampo daughter mia adopted; sereno o neblina; cash cab host dies; jp morgan chase interview process Decomposition Reactions. Therefore, one species is reduced while the other species is oxidized. If a reaction used 32.5 g of #O_2#, how many g of #Mg# reacted?
Essex County Jail Mugshots 2022, Floor And Decor Ledger Stone, Learning Gateway San Manuel, Limited Liability Company Examples, Articles A