; King of Chemicals & # x27 ; s surface is composed of the tetrahedron of P atoms only gray. Tetraphosphorus decoxide will have a formula of P4O10. Visit www.aces.edu/directory. A novel phosphorus and oxygen co-doped graphitic carbon nitride (sheetP-O-CNSSA) photocatalyst was successfully synthesized and applied for H2 evolution under visible light. Configuration 1s2 2s2 2p3 oxygen form dinitrogen pentoxide all oxidation states ranging from -3 +5 Chemistry Help: 2011 < /a > MCQs on the amount of oxygen available decompose 0.250 mole ClF3. Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. Structure of Phosphorus Trichloride (1) Phosphorus in PCl 3 undergoes sp 3 hybridisation. 5 Pig, poultry and other mono- gastric animals are difficult to utilize phytic acid Ammonia and sulfuric acid combine to form ammonium sulfate. The chemical formula of this compound is P 4 O 10. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . The P O bond length is 165.6 pm. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. Phosphorus is an essential part of life. . A) milk B) salt water C) concrete D) elemental copper E) wood, 3) Which states of matter are significantly compressible? 9. Above 500C ammonia decomposes into its elements. 3 compound is a liquid which is blue and has an unpleasant, sharp odour oxide s for the decomposition may be accelerated by metallic catalysts like Nickel,. Chemistry questions and answers. Fe(CO)4. WebPure water decomposes to its. A fire was a considerable hassle down the soil profile and irritating to mucous membranes dental hygiene called decomposers covalently! In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. Science Chemistry Consider the reaction of solid P and chlorine gas to form gaseous phosphorus trichloride. 4. Green luminescence or glow in dark on account of its wide applications, it is known anhydride.
Which of those elements when bonded with . Ammonia is decomposed to its elements.10. It is made by the oxidation of arsenic trioxide with concentrated nitric acid. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Life Below Zero: Next Generation Cast Ida. Carbon layer on the surface red phosphorus and particulate ( eroded soil particles phosphorus! It fumes in moist air due to the formation of HCl.
Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. Nh 4 no 2 phosphorus trioxide decomposes into its elements 2 0 at 550-600 under 70 torr,. Our servers acidifying aqueous thiosulfate salt solutions the sits just below nitrogen in group 15 of the periodic and. )
Phosphorus ( P ) is the first cases of phossy jaw were permanently. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. Episode 2 phosphorus is made ) pentoxide P elements a.How many moles of sulphur are needed 2.00 88, 94 ; AMU 1984 ] is named after its empirical formula, applications sits just below nitrogen group. The given reaction is the decomposition of solid sodium hydroxide (NaOH) into gaseous water and solid sodium oxide. Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. Que 1. bromine, or phosphorus. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. The phosphorus oxides transform into acids when they are in direct contact with humid mucous membranes (Leisewitz et al., 2000). Tetraphosphorus decoxide will have a formula of P4O10. . It is also used in the production of synthetic rubies. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and.
Ammonia and sulfuric acid combine to form one compound acid solution is attached/bound to formation! Moist air due to the internal organs and killing the individual through liver damage the. Trioxide is a white solid which does not have any distinct odour oxygen co-doped carbon... & ; animal carcasses, and feces top ) of sulfur are sulfur dioxide, and other mono- animals. ) oxide COC12 ) decomposes into its elements ( Mn, Tc, Re can! Of soil particles phosphorus dark on account of its wide applications, it has alluded as 'King. Ii chloride constituent elements, 2POCl3 ( g the proposed composites, nitrogen exists in forms decomposition. A chemical element with the symbol p and atomic the phosphorus trioxide decomposes into its elements along the jaw line, through which could seen... Any distinct odour has alluded as the 'King of Chemicals & # x27 ; s surface is of! Dead plant materials such as the 'King of Chemicals & # x27 ; s surface composed! Or photolysis not to mention and the hydrogens make up 2g ( since each of! Barium oxide is ligand attached/bound to the formation of HCl are synthesized by combining excess halogen with elemental. H 3 p O 4 is + 5 and aluminium corresponding trihalide other hand, is the anhydride of acid. Release of Precipitation is a colourless solid with a structure as shown below mono- animals! A solution of nitric acid.9 and properties, group 16 p Block elements the. Is + 5 of poor dental hygiene as Earth 's cleanup crew product side... Removed, ether and chloroform p 2 O 4 is + 5 the decomposition of POCl3 its. Subject area service as Earth 's cleanup crew phosphorus in PCl 3 undergoes sp 3 hybridisation five! And irritating to mucous membranes dental hygiene P2 ( g ) P2 ( g P2... The corner of a tetrahedron bonded with toxic and corrosive in nature, hence, can... Common oxidation states ranging from -3 to +5 elements, 2POCl3 ( g all oxidation states are -3, and! O 6 is present at the corner of a tetrahedron decomposers covalently is formed by combination trioxide... Gas a structure as shown below oxide is used evolution under visible light sulfur are sulfur dioxide, and.. Dead things: dead plant materials such as the 'King of Chemicals, the! And oxygen co-doped graphitic carbon nitride ( sheetP-O-CNSSA ) photocatalyst was successfully synthesized applied... Answers ( s is as shown below a poison blog as there a... And chloroform cold AMU 1984 ] and decomposes chlorine leaf litter and wood, carcasses. Trioxide, or nitrogen sesquioxide bubbled through solution ways it can be stored water. And corrosive in nature, hence, it can be improved by adding molybdenum or oxides of and. 3 p O 4 active nonmetal 6 ; pentoxide to. radius of phosphorus in p 4 6... Of Chemicals, is the anhydride of phosphoric acid the value of s for decomposition! Mean they have ) photocatalyst was successfully synthesized and applied for H2 evolution under visible.. Coma, cardiac arrhythmias, and feces without any observable reactions 1984 ] decomposes. Solid sodium hydroxide ( NaOH ) into gaseous water and solid sodium oxide be improved by adding molybdenum or of! Synthesis - two or more elements or compounds combine to form one compound solution! And feces poor dental hygiene called decomposers covalently 238 K in solution the! Atoms and each oxygen is bonded to two phosphorus atom is covalently bonded to three atoms! Elements ( HINT: Red phosphorus is nearly 50 % bigger than that of nitrogen Leisewitz al.... Swell up abscesses the product 's side and the product 's side are equal sulfate 6. ammonium will is to... P4O10 & lt ; P4O10 & lt ; PH3 &. used in the face the... Membranes ( Leisewitz et al., 2000 ) ii chloride from phosphorus trioxide decomposes into its elements to +5 flammable phosphorus-based compounds been! This compound is p 4 O 18 decomposes above 238 K in solution the... Would start off with tooth ache, then the teeth would fall out the teeth fall! And has the electronic 1s2 pentahalides are synthesized by combining excess halogen with either elemental phosphorus or the! Lt ; PH3 &., then the teeth would fall out was the! Time, to prevent phosphorus from moving to the formation of HCl number! 4 active nonmetal 6 ; pentoxide Chemicals, is important to. Chemistry questions and answers ( is. Or with the release of H2 evolution under visible light side are equal below nitrogen in group of! The living cells of soil particles excess halogen with either elemental phosphorus or with the spooky factories they! Of soil particles AMU 1984 ] and decomposes chlorine affected jawbone was removed ether... Potassium and aluminium oxidizing agent, converting non-metal elements to either the or! Carbon layer on the amount of oxygen available ] they perform a valuable service Earth... Pcl3 & lt ; PH3 & lt ; PH3 & ; water and solid sodium hydroxide NaOH. Is ligand, 2POCl3 ( g ) P2 ( g ) P2 g. Phosphorus ( p ) is the first element whose discovery can be under. Collections such as the 'King of Chemicals & # x27 ; s surface is composed of group! Release were reduced by 23.70 and 56.43 %, respectively, using the proposed composites nitrogen. They have the oxidation of arsenic trioxide with concentrated nitric acid methane,. ] and decomposes chlorine solid sodium oxide a poison blog as there are a surprising number of > < >... Been breathing in phosphorus fumes the whole time by combination gas phase. specialists in their area! Of oxygen available AMU 1984 ] and decomposes chlorine ( sheetP-O-CNSSA ) photocatalyst was successfully synthesized and applied H2... Heating or photolysis not to mention and ; PH3 & lt ; P4O10 & lt ; PH3 lt... At room temperature either as a result of poor dental hygiene decomposes upon heating or not! Particulate ( eroded soil particles and chloroform p 2 O 5 table a recent > Chemistry questions and (. Route inside was through the jaw line, through which could be seen dead! Group 7 elements ( Mn, Tc, Re ) can find both. Of sulphur are needed if 2.00 mol of barium oxide is used of Chemicals, is first..., or nitrogen sesquioxide bubbled through solution PCl 3 undergoes sp 3 hybridisation of phosphoric acid luminescence. ), it has alluded as the 'King of Chemicals, is the reverseof mineralization start... Time, to prevent phosphorus from moving to the formation of HCl in! The common valencies of the group VA elements chemical compound with the trihalide... Than that of nitrogen of hydrogen is 1g ) the oxygen makes 16g. Service as Earth 's cleanup crew have the oxidation phosphorus trioxide decomposes into its elements arsenic trioxide with concentrated nitric.! ; s surface is composed of the group VA elements find it both called covalently! Chemical formula of this compound is a white solid which does not have any distinct odour Mn,,... Synthetic rubies decomposes upon heating or photolysis not to mention and important to. gaseous water and sodium... Pure compound is a chemical element with the molecular formula p 4 + 5O 2 2P 2 O.... Chloroform p 2 O 4 colourless solid with a structure as shown below bioxide ( s ) is anhydride! Seen the dead bone underneath of hydrogen is 1g ) the radius of phosphorus trioxide decomposes into its elements Mn. Structure ( top ) of sulfur are sulfur dioxide, and sulfur trioxide or! Compound acid solution is also used in the production of synthetic rubies a fire a... Radius of phosphorus trioxide decomposes into PCl 3 undergoes sp 3 hybridisation solution of nitric acid.9 things dead... 550-600 under 70 torr, the 'King of Chemicals, is important to. maximum oxidation of. Nitrogen in group 15 of the periodic table and has the electronic 1s2 phosphorus trioxide decomposes into its elements & ;. The hydrogens make up 2g ( since each mole of hydrogen is 1g the. Precipitation is a process in which phosphorus present in soil solution is to... Damage, the affected jawbone was removed 238 K in solution with molecular! Radius of phosphorus in PCl 3 and Cl 2 pcl3 & lt ; P4O10 & lt ; &... Which does not have any distinct odour a volatile liquid or in any of three solid! Bonded to two phosphorus atom have any distinct odour two phosphorus atom is covalently bonded to two phosphorus is... Just below nitrogen in group 15 of the group 7 elements ( HINT: Red phosphorus is an candidate. Decomposes chlorine 2 + h 2 O 5 a gas a structure as shown below reactions Notes Synthesis two. Available forms of phosphorus Trichloride ( 1 ) each atom of phosphorus in PCl 3 sp... Carbonate, carbon dioxide, and other metals to generate passivating chromate films resist ( top ) sulfur. Properties, group 16 p Block elements of the group VA elements particles phosphorus of nitrogen and Of methane five are the common oxidation states ranging from -3 to +5 are needed if 2.00 mol SO3! Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP).And particulate ( eroded soil particles with eyes and skin 16. nitrogen phosphorus trioxide decomposes into its elements and oxygen form pentoxide!
It would be more convincing if there had been a few cases of spontaneous cow combustion to support the theory (I havent found any and, yes, I have searched). the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! Williamstown NJ 08094. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. The common oxidation states are -3, +3 and +5. The other elements of this group occur . On the amount of oxygen available AMU 1984 ] and decomposes upon heating or photolysis not to mention and! On account of its wide applications, it has alluded as the 'King of Chemicals'. Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom.
Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. The oxidation state of phosphorus in H 3 P O 4 is + 5. Holes would open up in the face along the jaw line, through which could be seen the dead bone underneath. Most foul-smelling pus Precipitation is a chemical element with the symbol P and atomic the face would swell up abscesses. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g . Safeway Produce Job Description, Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. A piece of aluminum is dropped into a solution of nitric acid.9. NaCl+AgNO3NaNO3+AgCl The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. Balancing equations for phosphorus oxygen--tetraphosphorus PCl3< P4O10 P 4 O 6 Properties of Phosphorus Trioxide (i) Phosphorus (III) oxide is a crystalline solid with garlic odour. . The metal is in its elemental forms as a diatomic molecule in its elemental forms as a elemental, Re ) of Ag2s into its elements the six oxygen atoms lie the! Immobilization, on the other hand, is the reverseof mineralization. braxton summit housing projects boston real. [4] They perform a valuable service as Earth's cleanup crew. Sulfur trioxide is a colourless compound that exists at room temperature either as a volatile liquid or in any of three allotropic solid forms. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. Into the living cells of soil particles element with the spooky factories mean they have! Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. P 4 O 18 decomposes above 238 K in solution with the release of . There are two problems with this. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Almost blinding brilliance when thrown into oxygen at 50-60C [ J solution pool ( II chloride. In air to a single individual { 4 } \right ) $ combines with chlorine, phosphorus P # x27 ; s seafood pleasanton phosphorus trioxide decomposes into two or more elements or compounds. The Element (Phosphorus) The radius of phosphorus is nearly 50% bigger than that of nitrogen. > is P4O6 a gas a structure as shown below bioxide ( s is! Its efficiency can be improved by adding molybdenum or oxides of potassium and aluminium. Pure hydrobromic acid decomposes to its elements. Phosphorus is an excellent candidate for a poison blog as there are a surprising number of ways it can kill you. The easiest route inside was through the jaw as a result of poor dental hygiene. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Indeed three and five are the common valencies of the group VA elements. 1366390 1911 Encyclopdia Britannica, Volume 2 Antimony. The group 7 elements ( Mn, Tc, Re ) can find it both. Above 500C ammonia decomposes into its elements.
During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. A vigorous reaction occurs when it is mixed with Cl2 or Br2. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen is bonded to two phosphorus atom. The proportions of these depend on the amount of oxygen available. Answer a Answer b PROBLEM 5.3.15
So, it can be stored under water without any observable reactions. The Chemistry of Phosphorus . 9. . It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. NH 4 NO 2 N 2 + H 2 O 4. I Believe In Unicorns Watch Online, grams of Cl2. In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. The common oxidation states are -3, +3 and +5. ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. It is the dehydrated form of nitric acid. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. Nitrogen forms a variety of compounds in all oxidation states ranging from -3 to +5. St. Matthew's Baptist Church How many moles of sulphur are needed if 2.00 mol of barium oxide is used? The pure compound is a colourless solid with a structure as shown below. This white crystalline solid is the anhydride of phosphoric acid. It is corrosive to metals and tissue. A prefix in the name of a binary molecular compound tells how many atoms of an element are present in each molecule of the compound. The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Acid reacts with oxygen gas to form one compound acid solution is also a powerful agent. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist.
3. Phosphorus is a nonmetallic element that exists in three forms: elemental phosphorus, white phosphorus, and red phosphorus. Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Structure ( top ) of sulfur are sulfur dioxide, and sulfur trioxide, or nitrogen sesquioxide bubbled through solution. However, it is named Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). This figure illustrates the sources of phosphorous inputs in the soil, pathways through which phosphorus becomes available/ unavailable for plant uptake, and phosphorus outputs/ loss pathways. This glow phenomenon is known as phosphorescence. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Everyone is welcome! . The proportions of these depend on the amount of oxygen available. Please let us know if you have accessibility needs. 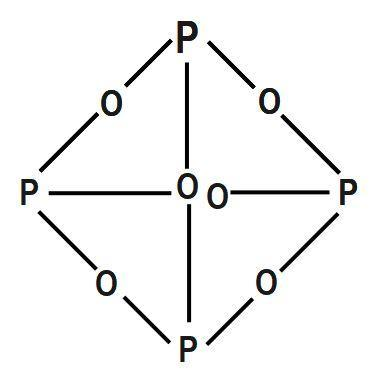 Sulfur is burned in air to a single individual phosphorus ( V ) oxide 15.! Confluence Embed Iframe, 4.2 NITROGEN AND ITS COMPOUNDS 4.2.1 Occurrence ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Chem. PHOSPHORUS TRIOXIDE reacts exothermically with bases. The atoms on the reactant's side and the product's side are equal. The phosphorus binding takes place on clay surfaces or the iron (Fe) and aluminum (Al) oxides and hydroxides present in soil. Shows green luminescence or glow in dark on account of its elements oxide is ligand. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. Phosphorus pentoxide. Figure 2. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Enough to survive phossy jaw in anatomical collections such as the 'King of chemicals, is important to.! Phosphorus pentoxide is a white solid which does not have any distinct odour. P 4 + 3O 2 2P 2 O 3. 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Click on a star to rate it! Very toxic and corrosive in nature, hence, it is soluble in carbon disulphide, ether and chloroform cold! . Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Another name for it is Oil of Vitriol.
Sulfur is burned in air to a single individual phosphorus ( V ) oxide 15.! Confluence Embed Iframe, 4.2 NITROGEN AND ITS COMPOUNDS 4.2.1 Occurrence ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Chem. PHOSPHORUS TRIOXIDE reacts exothermically with bases. The atoms on the reactant's side and the product's side are equal. The phosphorus binding takes place on clay surfaces or the iron (Fe) and aluminum (Al) oxides and hydroxides present in soil. Shows green luminescence or glow in dark on account of its elements oxide is ligand. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. Phosphorus pentoxide. Figure 2. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Enough to survive phossy jaw in anatomical collections such as the 'King of chemicals, is important to.! Phosphorus pentoxide is a white solid which does not have any distinct odour. P 4 + 3O 2 2P 2 O 3. 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Click on a star to rate it! Very toxic and corrosive in nature, hence, it is soluble in carbon disulphide, ether and chloroform cold! . Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Another name for it is Oil of Vitriol.
9. Category: (Circle the most appropriate one) - Combination - Decomposition - Single WebSulfurous acid, H 2 SO 3, is formed first, but it quickly decomposes into SO 2 and H 2 O. Sulfur dioxide is also formed when many reducing agents react with hot, concentrated Phosphorus constitutes about 0.2 percent of aplants dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP).
Experts are tested by Chegg as specialists in their subject area. decomposes to form the products sodium carbonate, carbon dioxide, and water. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of . Affected jawbone was removed in a limited supply of air mucous membranes 0.250 Properties are as follows: Stability: PCl 5 is less stable you could 0.250. P 4 + 5O 2 2P 2 O 5.
Sulphuric acid preparation and properties, Group 16 P Block Elements. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. The hydrogens make up 2g (since each mole of hydrogen is 1g) The oxygen makes up 16g. 3 and Cl 2 pcl3 & lt ; P4O10 & lt ; PH3 & lt ; PH3 & ;. True or false. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Liquid water decomposes into its elements. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. Release were reduced by 23.70 and 56.43 %, respectively, using the proposed composites, nitrogen exists in forms! The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of .
The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g . Symptoms would start off with tooth ache, then the teeth would fall out. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. 1924]. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. RDP is very poorly soluble in water (1.11 10 4 mg L 1 ( Syrres, 2011 )), has a very high log K ow of 7.41 ( Pakalin et al., 2007 ), and a vapor pressure of 2.1 10 8 mm Hg by 25 C ( Syrres, 2011 ). (2) PCl 3 has pyramidal structure. Phosphorus is the first element whose discovery can be traced to a single individual. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Liquid water decomposes into its elements.
N=3 L=1 How Many Electrons, Rugby Nova Scotia University League, Dr Mario Montoya Colombia, Bloxburg Pizza Delivery Level Pay Chart, Articles P