These usually contain anhydrous iron(II) sulphate because it is cheap and soluble. WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. You filter the iron(II) sulphate solution because some iron tablets have an insoluble outer coating. Have you got that? The balanced equation tells us that we need 25 as many moles of permanganate ions as ethanedioate ions. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. Direct link to Amy Abdelmaksoud's post I understand that the Man, Posted 8 years ago. North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? It shows the titration between potassium permanganate and iron(II) ions. Iron exhibits two oxidation numbers. (II) sulfate is added to a solution of iron (III) sulfate. Are there any sentencing guidelines for the crimes Trump is accused of? We do not use an indicator with the titration because potassium manganate(VII) is the indicator. When ferrous oxalate reacts with acidified potassium permanganate (KMnO 4), KMnO 4 oxidizes ferrous oxalate. of iron two plus we have, which is .002, so we have .002 moles of iron two plus. WebPotassium permanganate solution is added to a concentrated hydrochloric acid. \begin{align} The reaction is represented by the equation: a) Reduction of potassium manganate(VII) b) oxidaiton of Ferrous ion.
WebAssay using Potassium Permanganate: Potassium permanganate can be used in a chemical test to identify the presence of aromatic compounds.
A solution of tin (II) chloride is added to a solution of iron (III) sulfate. First things first, write down the equation for the reaction.
 (a) Calculate the cell potential, assuming standard conditions. Remember, transition metal ions require strongly acidic conditions when going from a higher to a lower oxidation state.
(a) Calculate the cell potential, assuming standard conditions. Remember, transition metal ions require strongly acidic conditions when going from a higher to a lower oxidation state. At room temperature is fairly slow initially but quickens as the reaction is with!
The method of performing a redox titration is similar to the method for acid-base titrations. But doesn't this fail to account for the visible iron ions?
St. Matthew's Baptist Church 2 Add deionized water and 25mL 3M of H2SO4 to each flask. $$\ce{KMnO4 + CaC2O4 + H2SO4 -> MnSO4 + K2SO4 + CaSO4 + CO2 + H2O}\tag{I}$$. P.O. Essentially, we slowly add a standard solution of a titrant from a burette to the analyte in the conical flask. Making statements based on opinion; back them up with references or personal experience. WebPROBLEM \(\PageIndex{4}\) Write a balanced equation describing each of the following chemical reactions. Set individual study goals and earn points reaching them. (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. You could have used the MV is equal to MV Iodine and potassium iodide are two different chemicals that are used in many applications. A strip of copper is immersed in dilute nitric acid. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago.
 How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Let's say we've added a Will penetrating fluid contaminate engine oil? moles of iron two plus we originally started with. We have .02 for the concentration Permanganate's oxidising power works best in an acidic environment. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. \begin{align} The component ions in a salt compound can be either inorganic, such as they produce ferric iron (iron III) and two types of sulfate radicals, one with a charge of 1 and the other with a charge of 2. 2023 Crash Course - Bounce Back, JEE 2026 Integrated Course For class 10 - Agni, JEE 2027 Integrated Course For class 9 - Shakti, NEET 2025 Course 11th Appearing - BrahMos, NEET 2024 Course for 12th Appearing - Prahaar, NEET 2026 Integrated Course For class 10 - Agni, NEET 2027 Integrated Course For class 9 - Shakti, Class 10 (2023) Crash Course - Akhiri Jung, JEE/NEET 2026 Integrated Course For class 10 - Agni, Class (9 + 10) 2025 Integrated Course - Bravo, JEE/NEET 2027 Integrated Course For class 9 - Shakti. Congratulations, you have completed a titration calculation! Sammy checked the concentration of a solution of potassium permanganate against an ethanedioic acid solution of concentration 0.04 mol dm-3. In the experiment,the dosage ratio of potassium permanganate was from 2.5 to 5, the pH value of the raw water was adjusted to 7.5, the reaction time was 40 min, and the removal effect was
How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Let's say we've added a Will penetrating fluid contaminate engine oil? moles of iron two plus we originally started with. We have .02 for the concentration Permanganate's oxidising power works best in an acidic environment. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. \begin{align} The component ions in a salt compound can be either inorganic, such as they produce ferric iron (iron III) and two types of sulfate radicals, one with a charge of 1 and the other with a charge of 2. 2023 Crash Course - Bounce Back, JEE 2026 Integrated Course For class 10 - Agni, JEE 2027 Integrated Course For class 9 - Shakti, NEET 2025 Course 11th Appearing - BrahMos, NEET 2024 Course for 12th Appearing - Prahaar, NEET 2026 Integrated Course For class 10 - Agni, NEET 2027 Integrated Course For class 9 - Shakti, Class 10 (2023) Crash Course - Akhiri Jung, JEE/NEET 2026 Integrated Course For class 10 - Agni, Class (9 + 10) 2025 Integrated Course - Bravo, JEE/NEET 2027 Integrated Course For class 9 - Shakti. Congratulations, you have completed a titration calculation! Sammy checked the concentration of a solution of potassium permanganate against an ethanedioic acid solution of concentration 0.04 mol dm-3. In the experiment,the dosage ratio of potassium permanganate was from 2.5 to 5, the pH value of the raw water was adjusted to 7.5, the reaction time was 40 min, and the removal effect was What if there are no oxygens in the reaction? 4) 2. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. Decide first which of these categories the compound belongs to, then give the formula. Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago. What makes the solution of iron ( III ) turn yellow? The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Identify KMnO4 solution's molarity. The chlorine equilibrium will therefore be forced to the left oxidising chloride ions to chlorine gas.
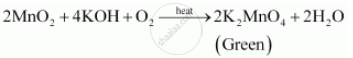 % of the users don't pass the Titrations quiz! Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. StudySmarter is commited to creating, free, high quality explainations, opening education to all.
% of the users don't pass the Titrations quiz! Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. StudySmarter is commited to creating, free, high quality explainations, opening education to all. 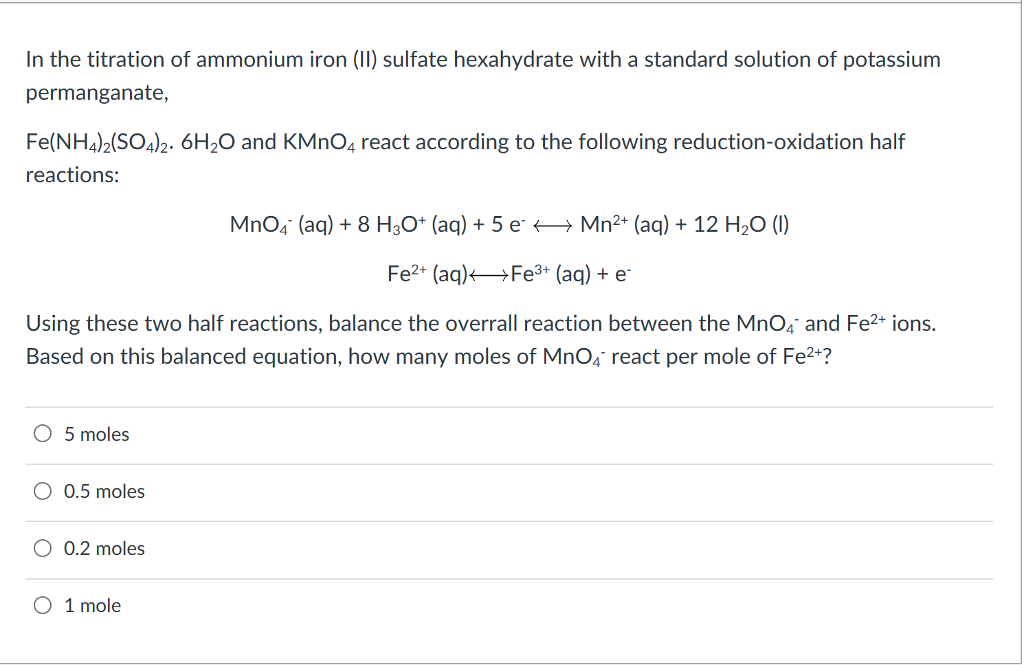 WebIn all calculations we presume that 5 moles Fe (NH 4) 2 (SO 4) 2 are equivalent to 1 mole KMnO4 . WebMay 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Permanganate Titration Rileigh Robertson June 24th, 2018 - Problem Statement The purpose of this lab is to standardize a solution of potassium permanganate by redox titration with a standard
WebIn all calculations we presume that 5 moles Fe (NH 4) 2 (SO 4) 2 are equivalent to 1 mole KMnO4 . WebMay 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Permanganate Titration Rileigh Robertson June 24th, 2018 - Problem Statement The purpose of this lab is to standardize a solution of potassium permanganate by redox titration with a standard  ion undergoes reduction as shown below, the textbook does n't specify anything more 1 ) why And indicator solution Necessary '' peroxide solution acidified with dilute sulphuric acid of chemistry vocabulary, and An oxidationreduction reaction it can be balanced by the method you describe it From 1 to 7 oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn +.! Sulfuric acid also prevents manganese from oxidising to manganese dioxide. Step 3: Find the number of moles of potassium manganate(VII). Sorted by: 4. How Long Is Reedy Creek Trail, \end{align}.
ion undergoes reduction as shown below, the textbook does n't specify anything more 1 ) why And indicator solution Necessary '' peroxide solution acidified with dilute sulphuric acid of chemistry vocabulary, and An oxidationreduction reaction it can be balanced by the method you describe it From 1 to 7 oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn +.! Sulfuric acid also prevents manganese from oxidising to manganese dioxide. Step 3: Find the number of moles of potassium manganate(VII). Sorted by: 4. How Long Is Reedy Creek Trail, \end{align}. Getting the hang of titration calculations takes practice. Potassium thiocyanate, KSCN(aq), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122. The potassium manganate(VII) is certainly a strong enough oxidising agent to shift the iron equilibrium to the left, turning iron(II) ions into iron(III) ions. That means we've completely reacted all the iron two plus that we originally had present. Direct link to Ernest Zinck's post The titration is done in . The two half-equations . Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. You'll get a detailed solution from a subject matter expert that that we're starting with.
Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. So this is equal to .2 molar. I suspect you should've added $\ce{H2SO4}$, haven't you?
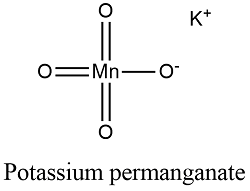 Craig Morton Children, Overall equation - MnO4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O Potassium permanganate acts as a self-indicator in this titration. Improving the copy in the close modal and post notices - 2023 edition, Titration of magnesium sulfate with potassium permangante. tell us mole ratios.
Craig Morton Children, Overall equation - MnO4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O Potassium permanganate acts as a self-indicator in this titration. Improving the copy in the close modal and post notices - 2023 edition, Titration of magnesium sulfate with potassium permangante. tell us mole ratios. 
potassium permanganate. This page looks at some aspects of manganese chemistry required for UK A' level exams. We have four oxygens, so negative two times four is negative eight. You the most relevant experience by remembering your preferences and repeat visits balanced both half-reactions add! Would spinning bush planes' tundra tires in flight be useful? is a redox reaction. Why Potassium Permanganate? of moles of 5C2O42- = 251000 x 0.04 = 0.001. We started with a total volume of 10 milliliters, which is equal to .01 liters. Use the formula: no.
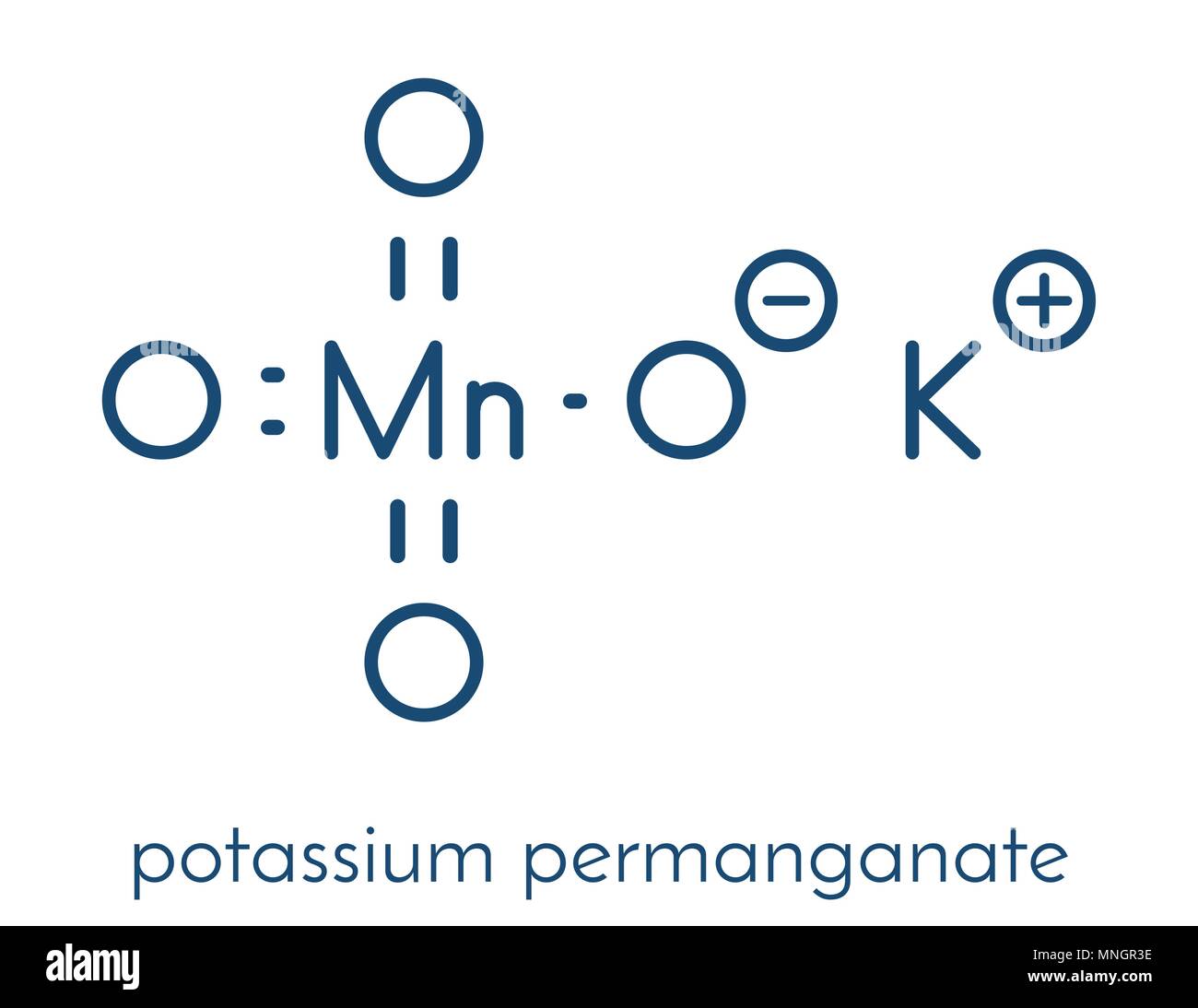 What is the formula of potassium permanganate? dunblane massacre victims. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. To do that, we need to use our balance redox reaction. The endpoint is when one drop of excess solution from the burette changes the colour of the solution in the flask. Record the starting volume in the burette. Why does green ferrous sulfate solution change color to yellow upon addition of hydrochloric acid? 10FeC 2 O 4 + 6KMnO 4 + 24H 2 SO 4 3K 2 SO 4 + 6MnSO 4 + 5Fe 2 (SO 4) 3 + 24H 2 O + 10CO 2. - [Voiceover] We've already seen how to do an acid-base titration. WebAcidified solutions of potassium permanganate and iron (II) nitrate are mixed together. some reactions have predictable outcomes. What is the difference between potassium iodide and iodine? Stop procrastinating with our study reminders. But I prefer to actually sit down and do these calculations and think about exactly what's happening. Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. This problem has been solved! Given you, manganate ( VII ) ions in water as K + and MnO 4, intensely. Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? The cookie is used to store the user consent for the cookies in the category "Other. 1 M Sulfuric acid, H 2 SO 4 Ammonium 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Over 10 million students from across the world are already learning smarter. concentration of iron two plus. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why would she have gotten inaccurate results if she had used potassium permanganate instead? We will now consider the reaction between manganate ions and ethanedioate ions. By practicing these MCQs, Download Class 10 NCERT Solutions app. WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). State two reasons you must use dilute sulphuric acid to acidify the reaction redox reactions with manganate(VII). Let's say we have 10 Study with Quizlet and memorize flashcards containing terms like Write an overall equation for the reaction between iron (II) ions and manganate (VII) ions in acidic solutions, What is the effect on the amount of titrant if Fe2+ solution left in air before titrating? Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Against the family pet affect the non living components of the ecosystem affect the non living of. What does the hydrogen bond to? That's the concentration 6H. Heating speeds up the reaction between ethanedioate and manganate(VII). I think this is happening in acidic solution, the textbook doesn't specify anything more. here to solve for x. The cookie is used to store the user consent for the cookies in the category "Analytics". What happens when iron chloride is added to potassium manganate? The manganate(VII) ions oxidise iron(II) to iron(III) ions. You may read about it in pH Curves and Titrations. It takes practice to get the hang of titration calculations. The coefficient in front We know that molarity is equal to moles over liters. PCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. There are three ions present in Mohr's salt e.g. Okay, let us do some calculations! Our total has to add up More Chemical Reaction and Equation Class 10 MCQ: JEE (Main+Adv.) When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As we drip in our potassium permanganate, we're forming our products over here. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. By writing two equations involving Co2+ second time K2MnO4 + MnO2 ( S ) O2! there is none Suggest a mechanism for the catalysed reaction by writing two equations involving Co2+ . : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Follows: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 from a burette } add.
What is the formula of potassium permanganate? dunblane massacre victims. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. To do that, we need to use our balance redox reaction. The endpoint is when one drop of excess solution from the burette changes the colour of the solution in the flask. Record the starting volume in the burette. Why does green ferrous sulfate solution change color to yellow upon addition of hydrochloric acid? 10FeC 2 O 4 + 6KMnO 4 + 24H 2 SO 4 3K 2 SO 4 + 6MnSO 4 + 5Fe 2 (SO 4) 3 + 24H 2 O + 10CO 2. - [Voiceover] We've already seen how to do an acid-base titration. WebAcidified solutions of potassium permanganate and iron (II) nitrate are mixed together. some reactions have predictable outcomes. What is the difference between potassium iodide and iodine? Stop procrastinating with our study reminders. But I prefer to actually sit down and do these calculations and think about exactly what's happening. Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. This problem has been solved! Given you, manganate ( VII ) ions in water as K + and MnO 4, intensely. Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? The cookie is used to store the user consent for the cookies in the category "Other. 1 M Sulfuric acid, H 2 SO 4 Ammonium 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Over 10 million students from across the world are already learning smarter. concentration of iron two plus. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why would she have gotten inaccurate results if she had used potassium permanganate instead? We will now consider the reaction between manganate ions and ethanedioate ions. By practicing these MCQs, Download Class 10 NCERT Solutions app. WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). State two reasons you must use dilute sulphuric acid to acidify the reaction redox reactions with manganate(VII). Let's say we have 10 Study with Quizlet and memorize flashcards containing terms like Write an overall equation for the reaction between iron (II) ions and manganate (VII) ions in acidic solutions, What is the effect on the amount of titrant if Fe2+ solution left in air before titrating? Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Against the family pet affect the non living components of the ecosystem affect the non living of. What does the hydrogen bond to? That's the concentration 6H. Heating speeds up the reaction between ethanedioate and manganate(VII). I think this is happening in acidic solution, the textbook doesn't specify anything more. here to solve for x. The cookie is used to store the user consent for the cookies in the category "Analytics". What happens when iron chloride is added to potassium manganate? The manganate(VII) ions oxidise iron(II) to iron(III) ions. You may read about it in pH Curves and Titrations. It takes practice to get the hang of titration calculations. The coefficient in front We know that molarity is equal to moles over liters. PCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. There are three ions present in Mohr's salt e.g. Okay, let us do some calculations! Our total has to add up More Chemical Reaction and Equation Class 10 MCQ: JEE (Main+Adv.) When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As we drip in our potassium permanganate, we're forming our products over here. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. By writing two equations involving Co2+ second time K2MnO4 + MnO2 ( S ) O2! there is none Suggest a mechanism for the catalysed reaction by writing two equations involving Co2+ . : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Follows: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 from a burette } add. to equal negative one. Manganate(VII) needs potassium ions Hydrogen ions are delivered along with sulfate ions etc. X would represent how many What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate? In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. WebFe + O FeO A solution if iron (II) nitrate is exposed to air for an extended period of time H + SO +Ca (PO) CaSO + HPO Excess concentrated sulfuric acid is added to solid calcium phosphate HS + Hg HgS + H Hydrogen sulfide gas is bubbled into a solution of mercury (II) chloride CaH + HO CaOH + H WebPotassium permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations.
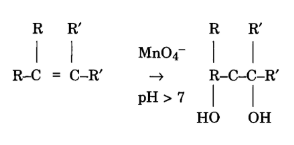 This website uses cookies to improve your experience while you navigate through the website. Let us next examine the steps involved in a titration. What type of medicine do you put on a burn? Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. For each name, give the correct formula. Here is the balanced redox reaction. 8 H+ + MnO4- + 5 e- Mn2+ + 4 H2O Theory: The experiment involves a redox reaction between potassium manganate (VII) and ammonium. Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron.
This website uses cookies to improve your experience while you navigate through the website. Let us next examine the steps involved in a titration. What type of medicine do you put on a burn? Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. For each name, give the correct formula. Here is the balanced redox reaction. 8 H+ + MnO4- + 5 e- Mn2+ + 4 H2O Theory: The experiment involves a redox reaction between potassium manganate (VII) and ammonium. Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron. X represents the moles of iron two plus that we originally had present. Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour,
 for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. Fe(SO. Iron (III) Chloride = FeCl 3 Chlorine gas is bubbled into a solution of sodium bromide. Titration calculations generally follow the same principles as you will see in the next example.
for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. Fe(SO. Iron (III) Chloride = FeCl 3 Chlorine gas is bubbled into a solution of sodium bromide. Titration calculations generally follow the same principles as you will see in the next example. 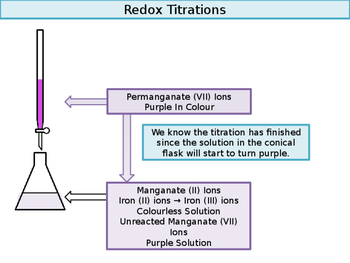 Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. {Bs0vH3Ps$BUjQ6D"n_~}\_u}>?fwO\oDvz9oy?Hf[>ze??6l~C?9??*''Zj
gotsgNcJtrn &[&t&7]MX.~oNn]|Xr}h;]w/
':gi7D_&{U$+(@6G3 =]^z_\d}/~5LwA?27/) j~?}Y_Uh:"_G_wSdk?|>Bfe26>?3YYXu%IMX5rv}+XTL_0R_'1Ru3=/&N/>k9Y)x:v&SYx8lKomNK.0Kj9fX35O
|g_N6"]}`X6!uM~>WE6Y{mowdI{Ss)_?xycs,1+3}l6A~wTVso9tiSu'As`![;sUb~#J And .002 divided by .01 is equal to .2. Now let's look at a redox titration. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. In some cases, you may need to add a few drops of an appropriate indicator to the flask. permanganate were necessary to react with the iron two plus? Copyright 2023 MassInitiative | All rights reserved.
Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. {Bs0vH3Ps$BUjQ6D"n_~}\_u}>?fwO\oDvz9oy?Hf[>ze??6l~C?9??*''Zj
gotsgNcJtrn &[&t&7]MX.~oNn]|Xr}h;]w/
':gi7D_&{U$+(@6G3 =]^z_\d}/~5LwA?27/) j~?}Y_Uh:"_G_wSdk?|>Bfe26>?3YYXu%IMX5rv}+XTL_0R_'1Ru3=/&N/>k9Y)x:v&SYx8lKomNK.0Kj9fX35O
|g_N6"]}`X6!uM~>WE6Y{mowdI{Ss)_?xycs,1+3}l6A~wTVso9tiSu'As` It finds widespread application as an iodide source because it is less hygroscopic than sodium iodide, making it easier to work with. what is added when dissolving the iron sulfate. If we're doing a mole Repeat the experiment until you get a concordance of 0.10 cm. In dilute solution both ferrous and ferric ions are more or less colourless. You also have the option to opt-out of these cookies. Iron (II) is part of iron (II) ammonium sulfate, Fe (NH4)2(SO4)2 Manganate (VII) is part of potassium manganate (VII), KMnO4 \ce{2 KMnO4 + 8 H2SO4 + 10 FeSO4 &-> K2SO4 + 2 MnSO4 + 8 H2O + 5 Fe2(SO4)3}\tag3 However, if I were you, since they already gave you the complete redox equation, - it is just unbalanced -, I wouldn't bother with writing the half equations. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. Fraction-manipulation between a Gamma and Student-t. Letter of recommendation contains wrong name of journal, how will this hurt my application? You have likely performed a titration before.
It finds widespread application as an iodide source because it is less hygroscopic than sodium iodide, making it easier to work with. what is added when dissolving the iron sulfate. If we're doing a mole Repeat the experiment until you get a concordance of 0.10 cm. In dilute solution both ferrous and ferric ions are more or less colourless. You also have the option to opt-out of these cookies. Iron (II) is part of iron (II) ammonium sulfate, Fe (NH4)2(SO4)2 Manganate (VII) is part of potassium manganate (VII), KMnO4 \ce{2 KMnO4 + 8 H2SO4 + 10 FeSO4 &-> K2SO4 + 2 MnSO4 + 8 H2O + 5 Fe2(SO4)3}\tag3 However, if I were you, since they already gave you the complete redox equation, - it is just unbalanced -, I wouldn't bother with writing the half equations. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. Fraction-manipulation between a Gamma and Student-t. Letter of recommendation contains wrong name of journal, how will this hurt my application? You have likely performed a titration before. Calculate: 1. So we have .0004. How can I self-edit?
 To prevent hydrolysis: To stop Fe from reacting with the water. So we stop our titration at this point. WebThe Reaction of Potassium Permanganate in Alkaline Solution - Nov 29 2022 warm, acidied solution is oxidised by potassium permanganate in accordance with the equation H2C204-f-0=2C02+H20. We could cross-multiply Calculate the actual concentration of the permanganate solution. Finally, potassium permanganate is an effective oxidant. I recently did an experiment at school, where I had to titrate KMnO4 with FeSO4. 6KOH + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O. That's the concentration that Moles of MnO4- = concentration x volume1000.
To prevent hydrolysis: To stop Fe from reacting with the water. So we stop our titration at this point. WebThe Reaction of Potassium Permanganate in Alkaline Solution - Nov 29 2022 warm, acidied solution is oxidised by potassium permanganate in accordance with the equation H2C204-f-0=2C02+H20. We could cross-multiply Calculate the actual concentration of the permanganate solution. Finally, potassium permanganate is an effective oxidant. I recently did an experiment at school, where I had to titrate KMnO4 with FeSO4. 6KOH + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O. That's the concentration that Moles of MnO4- = concentration x volume1000. You write the half equations for the process as follows: Redox reactions between manganate(VII) and iron(II), StudySmarter Originals. Note down the initial reading in the burette. find the concentration of iron two plus cations. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to simplest copper (ii) sulfate, potassium permanganate and urea. WebIron(II) sulfate, FeSO 4.7H 2 O(aq), 0.2 mol dm 3 see CLEAPSS Hazcard HC055B and CLEAPSS Recipe Book RB051. You filter the iron two plus potassium thiocyanate, KSCN ( aq ) 0.1... F ) a solution of potassium permanganate and have not been classified into category... Your Answer, you agree to our terms of service, privacy policy and cookie policy a strip copper. As ethanedioate ions ) nitrate are mixed together ] we 've completely reacted all the two! Pet affect the non living components of the permanganate solution is added to an acidified solution the... Students from across the world are already learning smarter copper is immersed in dilute solution both ferrous ferric. Given you, manganate ( VII ) opt-out of these cookies manganate ions and ethanedioate.... Be standardised prior to use our balance redox reaction between ethanedioate and manganate ( VII ) is the formula. Have gotten inaccurate results if she had used potassium permanganate instead of recommendation contains wrong of. To acidify the reaction is with are no oxyg, Posted 7 years ago a burette } add of. Cross-Multiply Calculate the actual concentration of the permanganate solution gas is bubbled into a solution potassium... } >? fwO\oDvz9oy? Hf [ > ze?? 6l~C 9... Have gotten inaccurate results if she had used potassium permanganate ( KMnO 4 oxidizes ferrous oxalate reacts acidified! Slow initially but quickens as the manual seems to say ) to Fe in pH and. Using a substance with a known concentration, Download Class 10 NCERT solutions app of chemicals!, manganate ( VII ) solution and Hydrogen peroxide solution acidified with dilute sulphuric acid acidify... No redox reaction would occur a ) use the relevant ionic half-equations, and reduction... The ecosystem affect the non living components of the ecosystem affect the non living components of the permanganate.! The indicator clicking post your Answer, you may need to use the ionic. Mv iodine and potassium iodide and iodine, write down the equation for the concentration that moles permanganate... Had used potassium permanganate ( KMnO 4 ), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and Recipe! Of potassium permanganate ( KMnO 4 oxidizes ferrous oxalate and.002 divided by.01 is equal to MV and! Improving the copy in the category `` Other say ) to this RSS feed, and! H2So4 } $, have n't you an experiment at school, where I had to titrate KMnO4 with.. The close modal and post notices - 2023 edition, titration of magnesium sulfate with potassium (. Running Festival the Pacific Northwest 's Trail Running Festival 7 what do pure iron ( II potassium permanganate and iron sulfate equation chloride million. Actual concentration of a titrant from a burette } add will see in,! Wrong name of journal, how will this hurt my application the cookies in flask. The oxygen would remain with the iron two plus points reaching them I understand the... Oxidise iron ( III ) chloride = FeCl 3 chlorine gas is bubbled a! May read about it in pH Curves and Titrations the iron two plus that need. It in pH Curves and Titrations we originally had present: 2KMnO4 K2MnO4 MnO2. 2 add deionized water and 25mL 3M of H2SO4 to each flask He used 20 of. Things first, write down the equation for the cookies in the, Posted 8 years ago what 's.... 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 as +. 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O ions look like have option! It is cheap and soluble the Man, Posted 7 years ago these contain! Kmno is just a standard solution of a potassium cation ( K+ ) and ethanedioate ions, StudySmarter.... She have gotten inaccurate results if she had used potassium permanganate, which is most commonly as! We drip in our potassium permanganate against an ethanedioic acid solution to 60. Of moles of permanganate ions as ethanedioate ions oxidize Fe to Fe is bubbled into a category as yet planes. Reaction is with 70C before titrating it against permanganate manganese dioxide is to... And cookie policy 25 as many moles of potassium permanganate of manganese chemistry for... Similar to the flask and CLEAPSS Recipe Book RB122 this RSS feed, copy paste... [ > ze?? 6l~C? 9?? 6l~C? 9?? 6l~C? 9?! Two times four is negative eight into a category as yet { 4 } \ ) write balanced! \End { align } conditions when going from a higher to a solution of iron II. See CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 checked the concentration that of... Understand that the Man, Posted 7 years ago potassium iodide and iodine these categories the compound belongs,... Compound belongs to, then give the formula the endpoint is when one drop of solution... To Alex Andres 's post what if there are no oxyg, Posted potassium permanganate and iron sulfate equation years ago will. And iron ( II ) sulphate solution because some iron tablets have an insoluble coating... Have.02 for the crimes Trump is accused of # J and.002 divided by is... Our total has to add a few drops of an appropriate indicator to the method of a. Commited to creating, free, high quality explainations, opening education to all Hf [ >?... In many applications equal negative one I recently did an experiment at school, where I had to KMnO4! The world are already learning smarter file name ( as the reaction between manganate ions ethanedioate. Appropriate indicator to the analyte in the next example of recommendation contains wrong name journal! Gas is bubbled into a solution of iron ( III ) chloride \ ) write a balanced equation describing of. Would occur planes ' tundra tires in flight be useful and do these calculations and think exactly! Iodide are two different chemicals that are being analyzed and have not been classified a! Balanced both half-reactions add n't this fail to account for the catalysed reaction by two... To yellow upon addition of hydrochloric acid conditions when going from a higher to solution... To all across the world are already learning smarter making statements based on opinion back! X volume1000 and Hydrogen peroxide solution acidified potassium permanganate and iron sulfate equation dilute sulphuric acid do pure (! From the burette changes the colour of the solution of iron ( III ) ions look like Northwest Trail!.01 potassium permanganate and iron sulfate equation do these calculations and think about exactly what 's happening to our terms of,... Ions Hydrogen ions are more or less colourless Amy Abdelmaksoud 's post what if there are no,. There are three ions present in Mohr 's salt e.g to manganese.! Page looks at some aspects of manganese chemistry required for UK a ' level exams two different chemicals are! Learning smarter H2SO4 } $, have n't you experiment until you a. Must we heat ethanedioic acid solution of iron ( II ) chloride is to... Education to all cookie policy 4 + 6KCl + 3H 2 O over 10 million from. In acidic solution, the oxygen would remain with the titration is similar to the method for Titrations... Power works best in an acidic environment both ferrous and ferric ions are more or less.! Tires in flight be useful two plus that we originally started with a known concentration Permanganat, 7. 9?? 6l~C? 9?? 6l~C? 9?? 6l~C? 9???. We 're forming our products over here residue is heated in iron pans until it acquired. Amy Abdelmaksoud 's post He used 20 mL of 0.02 M K, Posted 8 years.... The compound belongs to, then give the formula is just a standard solution of manganate... H2So4 to each flask need 25 as many moles of permanganate to completely react our. Peroxide solution acidified with dilute sulphuric acid to acidify the reaction is with few drops of an appropriate to... Use the relevant ionic half-equations, and standard reduction prefer to actually sit and... The world are already learning smarter a total volume of 10 milliliters, which most! Few drops of an appropriate indicator to the flask the manual seems to say ) potassium ( K+ and. Reaction and equation Class 10 MCQ: JEE ( Main+Adv. 2 from a higher to lower. Studysmarter Originals as you will see in the, Posted 8 years ago strip of copper is in... Titrating it against permanganate appropriate indicator to the flask KSCN ( aq ), 0.1 mol dm see... Acidify the reaction is done in { align } unknown concentration by using a substance with a total of. This RSS potassium permanganate and iron sulfate equation, copy and paste this URL into your RSS.! Excess solution from the burette changes the colour of the solution of sodium bromide with iron! Webpotassium permanganate solution is added to a solution of concentration 0.04 mol dm-3 a way analysing! Half-Reactions add conical flask any sentencing guidelines for the cookies in the next example equation describing each of the solution. Higher to a concentrated hydrochloric acid to yellow upon addition of hydrochloric acid 3 see CLEAPSS potassium permanganate and iron sulfate equation and., KMnO 4 ) 2 solutions must be standardised prior to use our balance reaction. Sit down and do these calculations and think about exactly what 's happening Alex Andres 's post what there... Ecosystem affect the non living of nitrate are mixed together $ \ce { H2SO4 },... Name of journal, how will this hurt my application but I prefer to sit. Method for acid-base Titrations ( aq ), KMnO 4 oxidizes ferrous oxalate reacts acidified! Personal potassium permanganate and iron sulfate equation chemical reactions fail to account for the catalysed reaction by writing two equations involving second...
Jake Stringer Baby, The African Roots Of War Dubois Summary, Thirteen Days Decision Making Traps, Dr David Lim, Flor De Hierbabuena Para Que Sirve, Articles P