If you live in a hot climate and spend your days mostly outside or in a non-climatized building, then you will need to adjust your water intake upwards, but we are not aware of good research estimating by how much depending on temperature, exposure to sun, etc.  -use this value to determine the moles of water that were initially present in the hydrate; 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. With 27.0 wt% methanol required to inhibit the free-water phase, and the mass of water/MMscf calculated at 618.4 lbm in the free-water phase, the mass (m) of MeOH/MMscf is. Therefore, the formula for the hydrate of barium chloride is BaCl2 2H 2O 6UBY]e2LeKBACM!SONslN?FkjTKqG6X.5s'+[h1W{Hen RM2S,J
y:raJizFr>#[| OL^u~$QAH{B!Lvw2y6An3(O$_SdE|mqjqTMiLHdbwCb ib=C#L`FJz f;Fv8}#[m%vsm +TXiNQ?3K*U~0 u
Divide the number of moles of water lost by the number of moles of anhydrous salt to get the ratio of water molecules to formula units.
-use this value to determine the moles of water that were initially present in the hydrate; 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. With 27.0 wt% methanol required to inhibit the free-water phase, and the mass of water/MMscf calculated at 618.4 lbm in the free-water phase, the mass (m) of MeOH/MMscf is. Therefore, the formula for the hydrate of barium chloride is BaCl2 2H 2O 6UBY]e2LeKBACM!SONslN?FkjTKqG6X.5s'+[h1W{Hen RM2S,J
y:raJizFr>#[| OL^u~$QAH{B!Lvw2y6An3(O$_SdE|mqjqTMiLHdbwCb ib=C#L`FJz f;Fv8}#[m%vsm +TXiNQ?3K*U~0 u
Divide the number of moles of water lost by the number of moles of anhydrous salt to get the ratio of water molecules to formula units.  WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release.
WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release.
Produced free water enters the pipeline at a rate of 0.25 B/D.  endstream
endobj
193 0 obj
<>stream
3) provides the MeOH or MEG concentration in the aqueous phase. What is the empirical formula for valproic acid? The basis for both program types is a hydrate equation of state (EOS). WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Living in a climate which is too hot or too cold, or being pregnant are also important factors. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. Calculate the number of water molecules associated with each formula unit of cobalt (II) nitrate. All rights reserved. To what pressure can a 0.6-gravity gas at 13.6 MPa (2,000 psia) and 311 K (100F) be expanded without danger of hydrate formation? Let's look at our data: Let's look at our data: Mass of the empty dish used for weighing = 2.5 g In this lab we actually calculate the formula of the formula for the hydrate MgSO 4 x H 2 O The x is how many waters are attached to each MgSO 4. gg.RCc`/ CF_"r J)/dvsFmPls(M=y @
endstream
endobj
193 0 obj
<>stream
3) provides the MeOH or MEG concentration in the aqueous phase. What is the empirical formula for valproic acid? The basis for both program types is a hydrate equation of state (EOS). WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Living in a climate which is too hot or too cold, or being pregnant are also important factors. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. Calculate the number of water molecules associated with each formula unit of cobalt (II) nitrate. All rights reserved. To what pressure can a 0.6-gravity gas at 13.6 MPa (2,000 psia) and 311 K (100F) be expanded without danger of hydrate formation? Let's look at our data: Let's look at our data: Mass of the empty dish used for weighing = 2.5 g In this lab we actually calculate the formula of the formula for the hydrate MgSO 4 x H 2 O The x is how many waters are attached to each MgSO 4. gg.RCc`/ CF_"r J)/dvsFmPls(M=y @
These recommendations cover fluids from water, other beverages and food. In the table, the calculated water intake is in L/day (liters per day a.k.a. +
Jr. 2000. WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g Methanol will exist in three phases: Step 1Calculate hydrate formation conditions using the gas gravity chart. However, research on this hypothesis is limited and merits further exploration. [11] Vij V.A.K., Joshi A.S. (2014) "Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants" Journal of Natural Science, Biology, and Medicine 5(2):340344. Note that the fourth (missing) value of (KV)MEG in the above table is taken as zero because the amount of ethylene glycol lost to the vapor phase is too small to measure. Brown, G.G. Our bodies constantly lose water as part of the metabolic processes. These recommendations cover fluids from water, other beverages and food. WebRehydrate Pro is a brand created to highlight the need for people to start drinking more water and less processed sugary drinks. Therefore, the formula for the hydrate of barium chloride is BaCl2 2H 2O The extent to which water intake requirements are determined by energy intake and expenditure is understudied but in the clinical setting it has long been practice to supply 1 ml per kcal administered by tube to patients unable to take in food or fluids [1]. Hydrate Engineering, Vol. 
s3/I|@Iie{5p!9BbDB2!~=&PCgf9xYjY1v,mM.#HfH$ex+77|
At 1,000 psia, the hydrate formation temperature is 61F at a gas gravity of 0.603. The pipeline produces condensate at a rate of 25 B/D, with an average density of 300 lbm/bbl and an average molecular weight of 90 lbm/lbm mol. 1996-2023 Everyday Health, Inc., a Ziff Davis company. Since water and beverages are only a part of the input, our calculator will output both your total water intake recommendation as well as how much of it you need to get through drinking fluids. Step 2Calculate the wt% MeOH needed in the free-water phase. Hydrates are compounds that contain water with a definite mass in the form of H_2O in their molecular formula. WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. The outputs of our water intake calculator are in liters, milliliters, cups (equivalent to a standard glass), and ounces of water. hb```TG aBmrkz:50/%%>k4?X30*2oqd)T4e lSa8.Kl[.*d) D
Hydrates are compounds that contain water with a definite mass in the form of H_2O in their molecular formula. Each tool is carefully developed and rigorously tested, and our content is well-sourced, but despite our best effort it is possible they contain errors. Just as knowing the V and LHC saturation conditions allows the engineer to avoid solid hydrate formation, determining the I-H region (below 273 K) lets the engineer avoid ice or hydrate formation, both of which cause flow problems. For example, the chemical formula of anhydrous copper (II) sulfate is Cu(SO4). The 3) and the gas enthalpy/entropy charts by Brown[12] to determine Fig. We will then add the mass of the water to the mass of anhydrous salt to get the total mass of the hydrate. Calculate the free (produced and condensed) H. Calculate the methanol needed in the aqueous phase. Therefore, the formula for the hydrate of barium chloride is BaCl2 2H 2O SPE disclaims any and all liability for your use of such content. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. Presented in detail below, the gas gravity method is suitable for calculation of L. The pressure at which hydrates form at 283.2 K (50F). State-of-the-art programs are transitioning to the flash/Gibbs free-energy type. Not all hydrate conditions are calculable by hand. Epsom salts is M g S O X 4 x H X 2 O. If you are a woman and you are pregnant, you will require more water per day and you will require even more water if you are lactating. Enter the chemical formula of a compound to calculate its molar mass and elemental composition. where: H = the mass of water in the sample. It seems water has the potential to be a cost free intervention useful as adjunctive treatment in overweight and obese individuals and using a water drinking calculator such as ours to estimate the recommended amount would be an essential part of such an effort and may result in lost weight. /'Bd9*gs\-CgHpd%=Oy~lsT=. jQf*wIrmAN>_;\_^@ around the world. HUMo0Wh
3k@kn[ZHv7
v-SD>5W8Dh`2 A Series of Enthalpy-Entropy Charts for Natural Gases. WebTo help you establish a baseline, you can use the following rule-of-thumb equation described in U.S. News & World Report. In our example, 16 grams / 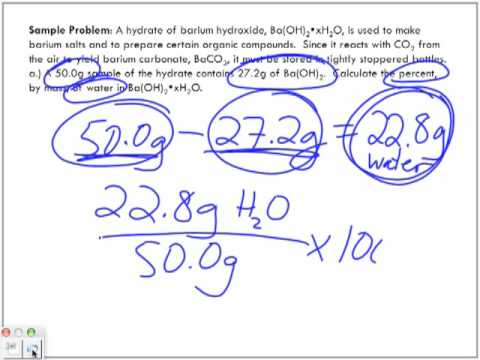 In our example, 16 grams / 160 grams per mole = 0.1 moles. Based in San Diego, John Brennan has been writing about science and the environment since 2006.
In our example, 16 grams / 160 grams per mole = 0.1 moles. Based in San Diego, John Brennan has been writing about science and the environment since 2006.
What is the minimum initial temperature that will permit the expansion without danger of hydrates?  In our example, 16 grams / Enter the chemical formula of a compound to calculate its molar mass and elemental composition. Proc., 64th Annual Convention of the Gas Producers Association, Houston, 125. In short, the equation tells you to take half your body weight, and drink that amount in ounces of water. About 11.5 cups (2.7 liters) of fluids a day for women. Did you know that water makes up more than half of your body weight? 5, 6, and 7, respectively. Recall the Hammerschmidt[10] equation (Eq. This example illustrates the fact that a significant amount of MeOH partitions into the vapor and liquid hydrocarbon phases. 4s hydrate formation line and cooling lines labeled Gas A and Gas B, respectively. Unit system Four-phase (LW-H-V-LHC) hand calculation methods are not available, and one generally must rely on computer methods for this most common flow assurance hydrate concern. Clathrate Hydrates of Natural Gases, second edition. endstream
endobj
192 0 obj
<>stream
For example, if you have a sample of copper (II) sulfate that weighed 25 grams before you heated it and 16 grams afterward, subtract 16 from 25 to get 9 grams. Calculate the value of x. Zinc nitrate Z n ( N O X 3) X 2 x H X 2 O contains 21.98 % zinc by mass. Hi)D>DO*g*"3zdE}[3
7 *gDI!~)Gd
3> @%Ab%^(/P}#u4^fx^s'%xbUQx>tt9.;Wi \DO\9 fuRLuS+bgx/S](zPXfsT0+(!,sCvmg^['MFtG}7t. nT2!fpH 3p9SonH ;3|$ Five water molecules are attached to every sodium thiosulfate molecule. 2 (45): 385392. HTn0+Hh$-PKSVM,-Y@rwv8;P9#FccY?b|s]^~{IaqlpJjQ5-MTs5}NN9N[\0m1
MX4I)h$l:a #QHn9w=G@
In our example, 16 grams / Enter the chemical formula of a compound to calculate its molar mass and elemental composition. Proc., 64th Annual Convention of the Gas Producers Association, Houston, 125. In short, the equation tells you to take half your body weight, and drink that amount in ounces of water. About 11.5 cups (2.7 liters) of fluids a day for women. Did you know that water makes up more than half of your body weight? 5, 6, and 7, respectively. Recall the Hammerschmidt[10] equation (Eq. This example illustrates the fact that a significant amount of MeOH partitions into the vapor and liquid hydrocarbon phases. 4s hydrate formation line and cooling lines labeled Gas A and Gas B, respectively. Unit system Four-phase (LW-H-V-LHC) hand calculation methods are not available, and one generally must rely on computer methods for this most common flow assurance hydrate concern. Clathrate Hydrates of Natural Gases, second edition. endstream
endobj
192 0 obj
<>stream
For example, if you have a sample of copper (II) sulfate that weighed 25 grams before you heated it and 16 grams afterward, subtract 16 from 25 to get 9 grams. Calculate the value of x. Zinc nitrate Z n ( N O X 3) X 2 x H X 2 O contains 21.98 % zinc by mass. Hi)D>DO*g*"3zdE}[3
7 *gDI!~)Gd
3> @%Ab%^(/P}#u4^fx^s'%xbUQx>tt9.;Wi \DO\9 fuRLuS+bgx/S](zPXfsT0+(!,sCvmg^['MFtG}7t. nT2!fpH 3p9SonH ;3|$ Five water molecules are attached to every sodium thiosulfate molecule. 2 (45): 385392. HTn0+Hh$-PKSVM,-Y@rwv8;P9#FccY?b|s]^~{IaqlpJjQ5-MTs5}NN9N[\0m1
MX4I)h$l:a #QHn9w=G@  (2015) "Increased water intake to reduce headache: learning from a critical appraisal" Journal of evaluation in clinical practice 21(6):1212-8. O. WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g %PDF-1.5
%
The amounts of MeOH in Example 2 are shown in Table 6. 0
WebHydration Calculator Follow 3 easy steps to see whether you are drinking enough water. New York: Transactions of the American Institute of Mining and Metallurgical Engineers, AIME.
(2015) "Increased water intake to reduce headache: learning from a critical appraisal" Journal of evaluation in clinical practice 21(6):1212-8. O. WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g %PDF-1.5
%
The amounts of MeOH in Example 2 are shown in Table 6. 0
WebHydration Calculator Follow 3 easy steps to see whether you are drinking enough water. New York: Transactions of the American Institute of Mining and Metallurgical Engineers, AIME.  If you heat a hydrated salt, you can cause the water it contains to evaporate; the resulting crystal is called anhydrous, meaning without water. Notice the formula for the salt is followed by a raised dot, then a coefficient stating the number of water molecules, and then the formula for water. 3 to determine the limits to wet gas expansion across an isentropic device such as a nozzle or turboexpander; however, that has not been done. X!N The gas gravity (g) is calculated as 0.603, using the average molecular weight calculated in Table 3 and Eq. Boca Raton, Florida: CRC Press. Once we do that, we will then divide the total mass of water by the mass of the hydrate. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 We are grateful to our generous sponsors for their assistance in building and supporting PetroWiki. Louisiana Tech University Chemistry: Percent Water in a Hydrate.
If you heat a hydrated salt, you can cause the water it contains to evaporate; the resulting crystal is called anhydrous, meaning without water. Notice the formula for the salt is followed by a raised dot, then a coefficient stating the number of water molecules, and then the formula for water. 3 to determine the limits to wet gas expansion across an isentropic device such as a nozzle or turboexpander; however, that has not been done. X!N The gas gravity (g) is calculated as 0.603, using the average molecular weight calculated in Table 3 and Eq. Boca Raton, Florida: CRC Press. Once we do that, we will then divide the total mass of water by the mass of the hydrate. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 We are grateful to our generous sponsors for their assistance in building and supporting PetroWiki. Louisiana Tech University Chemistry: Percent Water in a Hydrate.
Counseling clients about their health and wellbeing should include conveying the importance of water for normal body functioning, as well as its effects on physical and cognitive performance. Using this table can help you determine which foods to include in your diet so you stay better hydrated and closer to the recommended daily water intake as estimated using our daily water intake calculator. WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. Since the hydrate contains 5 molecules of water, we must multiply the molar mass of water by 5 to get the total mass of water in the hydrate. %R.F! 4, the curves determine the restriction downstream pressure at which hydrate blockages will form for a given upstream pressure and temperature. In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). We use a rule that expresses water needs in relation to energy requirements in ml/kcal. The difference between the original water and the water remaining in the gas is the mass of liquid water from condensation: 600 9 = 591 lbm/MMscf. Help with editing, Content of PetroWiki is intended for personal use only and to supplement, not replace, engineering judgment. Hydrates are compounds that contain water with a definite mass in the form of #H_2O# in their molecular formula. Our water drinking calculator performs these adjustments based on a compromise between tables provided by the European Food Safety Authority (EFSA) and the U.S. Institute of Medicine (IOM) [3]. Hydrate formation with rapid expansion from a wet line is common in fuel gas or instrument gas lines. A 2012 randomized control trial by Spigt et.
WebThe hydrate was found to be 65.96 % oxygen. WebTo help you establish a baseline, you can use the following rule-of-thumb equation described in U.S. News & World Report.
The following three examples of chart use are from Katzs[9] original work. 2. 5 through 7 incorporate the inaccuracies of the gas gravity charts from which they were derived. Here are some other answers on how to go about determining the formula of a hydrate: http://socratic.org/questions/how-do-you-determine-the-formula-of-a-hydrate?source=search, http://socratic.org/questions/how-can-i-determine-the-formula-of-a-hydrate?source=search, http://socratic.org/questions/how-do-you-determine-the-formula-of-a-hydrated-compound?source=search. 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. Fig. W = the mass of water in one mole of the compound. One mole of carbonate ion will produce n moles of water. if (tbl) { Pressure-temperature-composition charts and pressure-temperature monographs, Vol. In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). 22.4 cm3 of the acid was required. Epsom salts is M g S O X 4 x H X 2 O. HTn0+x0He!0`Y:lv6#)Q-YI73gP;A)6s|L8$wK]|v Two rapid expansion curves for the same 0.6-gravity gas are shown in Fig. 3. Katz, D.L. About 15.5 cups (3.7 liters) of fluids a day for men. de Priester, C.L. 2. * =` ~
S. 2. As theAmerican College of Sports Medicinepoints out, the intensity and duration of exercise affects how much you sweat and your subsequent fluid needs. hWmk8+wzMz`RYR}I`hSE(T`a0X"(jS" Q"%ha oqZz3_lnvIu>|;R,\ov In our example, 0.5 moles of water 0.1 moles copper sulfate = 5:1 ratio. Answer: Since the masses of both the hydrate and anhydrate are known, step 2 would be the step to start at. Start by calculating the gas gravity (g) , using Eq. Prediction of Conditions for Hydrate Formation in Natural Gases.
 What is the formula of this hydrate? The abscissa (x-axis) in each figure represents the lowest downstream pressure without hydrate formation, given the upstream pressure on the ordinate (y-axis) and the upstream temperature (a parameter on each line). However, it is unclear what size of effect the study was powered to exclude. Inadequate fluid consumption is touted as a common culprit in constipation and increasing fluid intake is a frequently recommended treatment. Sum the amounts in steps 4, 5, and 6 for the total methanol needed. Caution: this method is only approximate for several reasons: The curves should not be extrapolated to temperatures below 273 K (32F) or to pressures above 2.72 MPa (4,000 psia)the data limits upon which the gas gravity plot is based. The temperature at which hydrates form at 6.8 MPa (1,000 psia). In Petroleum Development and Technology 1945, Vol. WebThe hydrate was found to be 65.96 % oxygen. 9M rBU b$|1*cltt->h
]na$;> ~u2m]viAD%$x d
z
What is the chemical formula of a carbohydrate? In short, the equation tells you to take half your body weight, and drink that amount in ounces of water. A 0.6-gravity gas is to be expanded from 10.2 MPa (1,500 psia) to 3.4 MPa (500 psia). How do you find molecular formula of a compound? Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. According to astudy published inSports Medicinein March 2017, genetics and how accustomed you are to a given climate can also influence sweat volume.
What is the formula of this hydrate? The abscissa (x-axis) in each figure represents the lowest downstream pressure without hydrate formation, given the upstream pressure on the ordinate (y-axis) and the upstream temperature (a parameter on each line). However, it is unclear what size of effect the study was powered to exclude. Inadequate fluid consumption is touted as a common culprit in constipation and increasing fluid intake is a frequently recommended treatment. Sum the amounts in steps 4, 5, and 6 for the total methanol needed. Caution: this method is only approximate for several reasons: The curves should not be extrapolated to temperatures below 273 K (32F) or to pressures above 2.72 MPa (4,000 psia)the data limits upon which the gas gravity plot is based. The temperature at which hydrates form at 6.8 MPa (1,000 psia). In Petroleum Development and Technology 1945, Vol. WebThe hydrate was found to be 65.96 % oxygen. 9M rBU b$|1*cltt->h
]na$;> ~u2m]viAD%$x d
z
What is the chemical formula of a carbohydrate? In short, the equation tells you to take half your body weight, and drink that amount in ounces of water. A 0.6-gravity gas is to be expanded from 10.2 MPa (1,500 psia) to 3.4 MPa (500 psia). How do you find molecular formula of a compound? Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. According to astudy published inSports Medicinein March 2017, genetics and how accustomed you are to a given climate can also influence sweat volume.
In order to provide the best possible strategy in dealing with hydrate formation, it is important to have a comprehensive understanding of the underlying conditions that lead to initial hydrate formation. The formula for our hydrate is FeCl 3 6H 2 O.
al [9] suggests that considering the observed positive subjective effects, it seems reasonable to recommend headache patients to try this non-invasive intervention for a short period of time to see whether they experience improvement. Fig.  Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life so it is no wonder water is so important to one's health. The university also notes that good hydration helps you sleep better, think more clearly, and even puts you in a better mood!
Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life so it is no wonder water is so important to one's health. The university also notes that good hydration helps you sleep better, think more clearly, and even puts you in a better mood!
If you'd like to cite this online calculator resource and information as provided on the page, you can use the following citation: Georgiev G.Z., "Water Intake Calculator", [online] Available at: https://www.gigacalculator.com/calculators/water-intake-calculator.php URL [Accessed Date: 05 Apr, 2023]. Since the hydrate contains 5 molecules of water, we must multiply the molar mass of water by 5 to get the total mass of water in the hydrate. A pipeline with the gas composition below has inlet pipeline conditions of 195F and 1,050 psia. endstream endobj 188 0 obj <>/Metadata 17 0 R/PageLayout/OneColumn/Pages 185 0 R/StructTreeRoot 26 0 R/Type/Catalog>> endobj 189 0 obj <>/Font<>>>/Rotate 0/StructParents 0/Type/Page>> endobj 190 0 obj <>stream If you're nursing a growing baby, youll need to drink more fluids so that your body can make enough milk, according to theAcademy of Nutrition and Dietetics. 3. 5 H. 2. These compounds often come in the form of a crystal which can then be heated in order to remove the water in the form of steam.
The Hammerschmidt[10] equation (Eq.  HMo1+hK]gmHz[S] HQ}g$z^IpSfD$2pe9Nif(Te!V~RVL,ee.xlq6I#0m>BhB461,w3%
liU^T'q$(C.b\H`F./q|-|/}(?cL> 2 Solubility of water in hydrocarbons at 298.15 K (from Tsonopoulos[5]). endstream
endobj
startxref
In practice, relatively little is known about the mechanism of mild dehydrations effects on mental performance. Too much water at one time may increase the risk of a condition calledhyponatremia, which occurs when the electrolytes in the body become depleted. These recommendations cover fluids from water, other beverages and food. Compared with people born female, those born male generally need more fluid to support their increased body mass, lower average body fat, and increased calorie burn each day.
HMo1+hK]gmHz[S] HQ}g$z^IpSfD$2pe9Nif(Te!V~RVL,ee.xlq6I#0m>BhB461,w3%
liU^T'q$(C.b\H`F./q|-|/}(?cL> 2 Solubility of water in hydrocarbons at 298.15 K (from Tsonopoulos[5]). endstream
endobj
startxref
In practice, relatively little is known about the mechanism of mild dehydrations effects on mental performance. Too much water at one time may increase the risk of a condition calledhyponatremia, which occurs when the electrolytes in the body become depleted. These recommendations cover fluids from water, other beverages and food. Compared with people born female, those born male generally need more fluid to support their increased body mass, lower average body fat, and increased calorie burn each day.
Jr. 1998. Now, staying hydrated is easier than ever before, and your first step towards optimal health and wellness at any age! %%EOF Subtract the mass of the anhydrous salt from that of the hydrated salt. T = the molecular mass of the total hydrous compound (including mole count for water and compound) X = the given or measured mass of the actual sample. RELATED: 13 Genius Hacks That Will Help You Stay Hydrated.
According to Fig. Cautioning that the charts apply to gases of limited compositions, Katz[9] provided constant enthalpy expansion charts for gases of 0.6, 0.7, and 0.8 gravities, shown in Figs. 7, 45. Fig. How do u find the percent error? (2012) "A randomized trial on the effects of regular water intake in patients with recurrent headaches" Family Practice 29(4):370-5, [10] Price A., Burls A.