Using the 1,3-diaxial energy values given in the previous sections we can calculate that the conformer on the right is (7.6 kJ/mol - 2.0 kJ/mol) 5.6 kJ/mol more stable than the other. All of these systems usually form chair conformations and follow the same steric constraints discussed in this section. What is the most stable conformation of glucose? Why is Equatorial methylcyclohexane more stable? If the substituents are the same, there will be equal 1,3-diaxial interactions in both conformers making them equal in stability. Based on the table above, cis-1,4-disubstitued cyclohexanes should have two chair conformations each with one substituent axial and one equatorial. 4: Organic Compounds- Cycloalkanes and their Stereochemistry, { "4.01:_Naming_Cycloalkanes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The key difference between axial and equatorial position is that axial bonds are vertical while equatorial bonds are horizontal. This increase in the potential energy is known as the torsional strain. Thus, the staggered conformation is more stable than the eclipsed conformation because staggered conformation has no torsional strain. Solving for the equilibrium constant K shows that the equatorial is preferred about 460:1 over axial. Your textbook may offer you some hints for how to draw chairs. Each program has more options for drawing bonds than discussed here. Both chair conformations have one axial substituent and one equatorial substituent. WebIt turns out that it's going to be way more stable in the equatorial position. Thus, the equilibrium between the two conformers does not favor one or the other. There are only two possible relationships which can occur between ring-flip chair conformations: 1) AA/EE: One chair conformation places both substituents in axial positions creating 1,3-diaxial interactions. The left structure has 3 equatorial substituents while the structure on the right only has two equatorial substituents.
Any time you flip, you're going to be giving something in the axial position an opportunity to become equatorial. (I thank M Farooq Wahab, Chemistry, Univ Karachi, for suggesting that this article be noted here.). The equatorial positions are going to face slightly opposite to the axial.  Equatorial bonds will be roughly in the plane of the cyclohexane ring (only slightly up or down).
Equatorial bonds will be roughly in the plane of the cyclohexane ring (only slightly up or down).  In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are in bold. Since there are two equivalent chair conformations of cyclohexane in rapid equilibrium, all twelve hydrogens have 50% equatorial and 50% axial character. A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. 3.
In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are in bold. Since there are two equivalent chair conformations of cyclohexane in rapid equilibrium, all twelve hydrogens have 50% equatorial and 50% axial character. A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial. 3.
Equatorial groups are approximately horizontal, but actually somewhat distorted from that (slightly up or slightly down), so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5. Because the methyl group is larger and has a greater 1,3-diaxial interaction than the chloro, the most stable conformer will place it the equatorial position, as shown in the structure on the right. There will be three of each type. Especially when you put large groups there, you do not want to be in the axial position. Basically, we've got our axial positions and our equatorial positions. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. The Lower The Number, The More Stable It is. There is more room in the equatorial positions (not easily seen with these simple drawings, but ordinary ball and stick models do help with this point). The other conformer places both substituents in equatorial positions creating no 1,3-diaxial interactions. 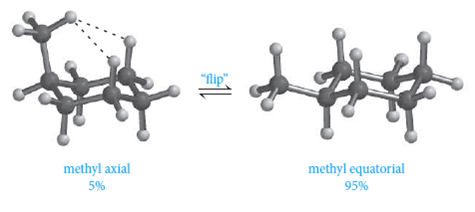 The solid (dark) "up wedge" I used is certainly common. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. It is located directly below the tool button for ordinary C-C bonds. WebAxial and equatorial are types of bonds found in the chair conformation of cyclohexane; The chair conformation is the most stable conformation of cyclohexane; Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring; The bond angles in this conformation are 110.9 When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. It is very common to confuse the two. In the next section will discuss the energy differences between these two possible conformations.
The solid (dark) "up wedge" I used is certainly common. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring. It is located directly below the tool button for ordinary C-C bonds. WebAxial and equatorial are types of bonds found in the chair conformation of cyclohexane; The chair conformation is the most stable conformation of cyclohexane; Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring; The bond angles in this conformation are 110.9 When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. It is very common to confuse the two. In the next section will discuss the energy differences between these two possible conformations.
Because the most commonly found rings in nature are six membered, conformational analysis can often help in understanding the usual shapes of some biologically important molecules. sketch the shorthand structure of cyclohexane, with axial and equatorial hydrogen atoms clearly shown and identified. WebIn cyclohexane, the equatorial position is energetically favored over the axial position. In the second set, one substituent is down and the other is up. When in the equatorial position, the methyl group is pointing up and away from the rest of the ring, eliminating the unfavorable 1,3-diaxial interaction. The wedges are available from the second toolbar across the top. The C-C-C bonds are very similar to 109.5o, so they are almost free from angle pressure. (Or rather: Where you minimize the energy according to the A Value ).  Each carbon has an axial and an equatorial bond. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle Each face of the cyclohexane ring has three axial and three equatorial bonds. Although the conformation which places the methyl group in the equatorial position is more stable by 7 kJ/mol, the energy provided by ambient temperature allows the two conformations to rapidly interconvert. That means that my equatorial position should face slightly down.
Each carbon has an axial and an equatorial bond. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle Each face of the cyclohexane ring has three axial and three equatorial bonds. Although the conformation which places the methyl group in the equatorial position is more stable by 7 kJ/mol, the energy provided by ambient temperature allows the two conformations to rapidly interconvert. That means that my equatorial position should face slightly down.
Which is the most stable chair conformation of cis 1/3 Dichlorocyclohexane? There are templates for simple chairs, without substituents (e.g., Fig 1B), and for chairs showing all the substituents (e.g., Fig 2B). This diequatorial conformer is the more stable regardless of the substituents. That means notice this one right here. When labeling the chair, it turns these two specifically to be both equitorial. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. 1 Answer. In cis-1,2-dimethylcyclohexane, both chair conformations have one methyl group equatorial and one methyl group axial. The figure below illustrates how to convert a molecular model of cyclohexane between two different chair conformations - this is something that you should practice with models. The chair conformation which places the larger substituent in the equatorial position will be favored. Which of these do you think is going to be the most stable? This conformer is (15.2 kJ/mol -3.8 kJ/mol) 11.4 kJ/mol less stable than the other conformer. It is located directly below the "Chain" tool button. A later chapter will discuss how many sugars can exist in cyclic forms which are often six remembered rings. When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring.
To Determine Chair Conformation Stability, Add Up The A-Values For Each Axial Substituent. WebThe most stable conformation is the one where the most bulky group is positioned equatorial. Because the axial is so Ring flip generates the less stable conformation with the large chloro group axial. Why? A similar conformational analysis can be made for the cis and trans stereoisomers of 1,3-dimethylcyclohexane. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. Hint: If you dont know what neopentyl is, its ok. Obviously it has 5 carbons, so keep that in mind when deciding equatorial preference! 5. One gauche-gauche conformer is particularly unfavorable because methyl groups are aligned with parallel bonds in close proximity. According to the guideline, the conformer with the larger substituent in equatorial is more stable because if the large group is axial, a stronger steric strain will be generated and it is less stable.
When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. WebAxial and equatorial are types of bonds found in the chair conformation of cyclohexane; The chair conformation is the most stable conformation of cyclohexane; Axial positions are perpendicular to the plane of the ring and equatorial positions are around the plane of the ring; The bond angles in this conformation are 110.9 The axial position is perpendicular to the plane of the ring of cyclohexane. Try to use the corners as much as possible. Based on the table above, trans-1,2-disubstitued cyclohexanes should have one chair conformation with both substituents axial and one conformation with both substituents equatorial. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. Make certain that you can define, and use in context, the key terms below. In either case, you can add, delete, or change things as you wish. Then looking at the "up" bond on each carbon in the cyclohexane ring they will alternate axial-equatorial-axial ect. After completing this section, you should be able to. So, despite having two axial groups, the first conformer is more as two chlorines do not bring as much steric interaction as the methyl group. Draw the two chair conformations for cis-1-ethyl-2-methylcyclohexane using bond-line structures and indicate the more energetically favored conformation. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In order to change the relationship of two substituents on a ring from cis to trans, you would need to break and reform two covalent bonds. Green = Equatorial. This is true for all monosubstituted cyclohexanes. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Each carbon has an axial and an equatorial bond. Also, remember that axial bonds are perpendicular with the ring and appear to be going either straight up or straight down. Due to the large number of bonds in cyclohexane it is common to only draw in the relevant ones (leaving off the hydrogens unless they are involved in a reaction or are important for analysis). Which is the most stable conformation of cyclohexane? WebAxial groups alternate up and down, and are shown vertical. When you feel the need, look around! Various kinds of stereo bonds (wedges and bars) are available by clicking the left-side tool button that is just below the regular C-C single bond button.
Which Teeth Are Normally Considered Anodontia? That means notice this one right here. WebIt turns out that it's going to be way more stable in the equatorial position. So the lowest energy conformer is the one where the most substituents are in equatorial position. Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle
Notice that a 'ring flip' causes equatorial hydrogens to become axial, and vice-versa. Note, that both methyl groups cannot be equatorial at the same time without breaking bonds and creating a different molecule. What is the order of stability of 1/4 Dimethylcyclohexane?
 To find the special templates for chairs, go to the Templates menu, choose Template Window, and then choose "Rings" from the drop-down menu near upper left.
To find the special templates for chairs, go to the Templates menu, choose Template Window, and then choose "Rings" from the drop-down menu near upper left.
tert-butyl > isopropyl > ethyl > methyl > hydroxyl > halogens. Because the axial is so much more torsionally strained with these H's here. And remember that we have our equatorial positions going slightly opposite. This conformation is called syn. To choose a type of stereo bond, click on the button and hold the mouse click; a new menu will appear to the right of the button. If you flip your chair, you also wind up flipping positions.  Even without energy calculations it is simple to determine that the conformer with both methyl groups in the equatorial position will be the more stable conformer. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. As always, the information provided on these pages in intended to help you get started.
Even without energy calculations it is simple to determine that the conformer with both methyl groups in the equatorial position will be the more stable conformer. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. As always, the information provided on these pages in intended to help you get started. 6.10A). When in an aqueous solution the six carbon sugar, g. lucose, is usually a six membered ring adopting a chair conformation. As predicted, one chair conformer places both substituents in the axial position and other places both substituents equatorial. As a consequence, the conformation in which the methyl group is in the equatorial position is more stable, by approximately 7 kJ/mol. No. That's what I'm trying to say. That one is facing up, that axial.
 We've got these ones on the positions and I just want to analyze the ones at the top. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. 2) Draw the two isomers of 1,4-dihydroxylcyclohexane, identify which are equatorial and axial. This is the #1 thing you need to know about cyclohexane.
We've got these ones on the positions and I just want to analyze the ones at the top. When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. 2) Draw the two isomers of 1,4-dihydroxylcyclohexane, identify which are equatorial and axial. This is the #1 thing you need to know about cyclohexane. 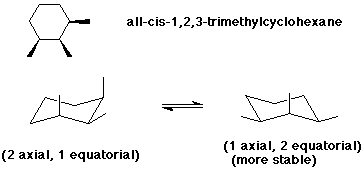 The equatorial positions are going to face slightly opposite to the axial. The latter is more stable (and energetically favorable) than the former. When looking at the two possible ring-clip chair conformations, one has all of the substituents axial and the other has all the substutents equatorial. That means that my equatorial position should face slightly down. Remember we have our axial positions, they're going straight up and down with the corners. With this it can be concluded that the bromine and chlorine substituents are attached in equatorial positions and the CH3 substituent is attached in an axial position. The gauche form is less stable than the anti form due to steric hindrance between the two methyl groups but still is more stable than the eclipsed formations. (Or rather: Where you minimize the energy according to the A Value ). As we would expect, the conformation with both methyl groups equatorial is the more stable one. The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction, Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris). A basic chair structure is provided on the default template bar that is shown. The terms cis and trans in regards to the stereochemistry of a ring are not directly linked to the terms axial and equatorial. Which of these do you think is going to be the most spread out?
The equatorial positions are going to face slightly opposite to the axial. The latter is more stable (and energetically favorable) than the former. When looking at the two possible ring-clip chair conformations, one has all of the substituents axial and the other has all the substutents equatorial. That means that my equatorial position should face slightly down. Remember we have our axial positions, they're going straight up and down with the corners. With this it can be concluded that the bromine and chlorine substituents are attached in equatorial positions and the CH3 substituent is attached in an axial position. The gauche form is less stable than the anti form due to steric hindrance between the two methyl groups but still is more stable than the eclipsed formations. (Or rather: Where you minimize the energy according to the A Value ). As we would expect, the conformation with both methyl groups equatorial is the more stable one. The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction, Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris). A basic chair structure is provided on the default template bar that is shown. The terms cis and trans in regards to the stereochemistry of a ring are not directly linked to the terms axial and equatorial. Which of these do you think is going to be the most spread out?
It is still possible to determine axial and equatorial positioning with some thought. As previously discussed, the axial methyl group creates 7.6 kJ/mol of steric strain due to 1,3-diaxial interactions. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. In fact, over 99% of this compound is going to exist in the equatorial position and less that 1% is going to exist in the axial position. That means that my equatorial position should face slightly down.